Potential Replacement of RDX with Low Sensitivities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthetic Advances in the C‐H Activation of Rigid Scaffold Molecules
Synthesis Review Synthetic Advances in the C‐H Activation of Rigid Scaffold Molecules Nitika Grovera Mathias O. Senge*a a School of Chemistry, Trinity College Dublin, The University of Dublin, Trinity Biomedical Sciences Institute, 152–160 Pearse Street, Dublin 2, Ireland [email protected] Dedicated to Prof. Dr. Henning Hopf Received: only recall the epic endeavors involved in Eaton’s cubane Accepted: Published online: synthesis,3 Parquette’s preparation of dodecahedrane, DOI: Prinzbach’s pagodane route thereto, or Maier’s synthesis of Abstract The remarkable structural and electronic properties of rigid non‐ tetra-tert-butyltetrahedrane.4 conjugated hydrocarbons afford attractive opportunities to design molecular building blocks for both medicinal and material applications. The bridgehead positions provide the possibility to append diverse functional groups at specific angles and in specific orientations. The current review summarizes the synthetic development in CH functionalization of the three rigid scaffolds namely: (a) cubane, (b) bicyclo[1.1.1]pentane (BCP), (c) adamantane. 1 Introduction 2 Cubane 2.1 Cubane Synthesis 2.2 Cubane Functionalization 3 BCP 3.1 BCP Synthesis 3.2 BCP Functionalization 4 Adamantane 4.1 Adamantane Synthesis 4.2 Adamantane Functionalization 5 Conclusion and Outlook Key words Cubane, bicyclo[1.1.1]pentane, adamantane, rigid scaffolds, CH‐ functionalization. Figure 1 The structures of cubane, BCP and adamantane and the five platonic hydrocarbon systems. The fascinating architecture of these systems significantly 1 Introduction differs from scaffolds realized in natural compounds. Hence, they attracted considerable attention from synthetic and The practice of synthetic organic chemistry of non-natural physical organic chemists to investigate their properties and to compounds with rigid organic skeletons provides a deep establish possible uses.3,5 Significantly, over the past twenty understanding of bonding and reactivity. -

Octanitrocubane - the Most Powerful Explosive of All?
Octanitrocubane - The most powerful explosive of all? Since the invention of gunpowder in 9th in the international edition of "Angewandte century China[1] and its subsequent distribution Chemie" in Jan. 2000[6]. over Europe and Arabia, humanity has since strived for new and better forms of explosives, What finally culminated in Eaton's and especially after the military relevance of such Zhang's successful synthesis had been a long compounds became obvious. It took, however, and tedious journey: For over 10 years several centuries until notable progress was synthetic chemists had worked on adding nitrate groups to cubane, one by one, until made, aside from improving the already [5] known gunpowder: In the middle of the 19th octanitrocubane was finally created . It is no century Nitroglycerine became one of the first wonder, that the final synthesis is not only very complex, but also difficult: No less than practical explosives not derived from [5] gunpowder thanks to the ongoing 40 steps were required to get to the final product, while only yielding it in sparse advancements in science and technology [4] (mainly in chemistry).[2] Since then the quantities . diversity of explosives has steadily increased. Now only one question stays unanswered: It is no wonder, that at some point, after the Can the "real" octanitrocubane stand up to its very elementary principles of explosives had "theoretical" twin? Does it exhibit the finally been discovered, scientists started to predicted properties? Well, yes and no: theorize, which compound might be the most While it is insensitive to shock and friction as powerful of all explosives. -

Synthesis and Characterization of Metal Complexes Containing
Wayne State University Wayne State University Dissertations 1-1-2010 Synthesis And Characterization Of Metal Complexes Containing Tetrazolate, Poly(tetrazolyl)borate, And Aryl Pentazole Ligands As High Energy Density Materials Dongmei Lu Wayne State University Follow this and additional works at: http://digitalcommons.wayne.edu/oa_dissertations Part of the Inorganic Chemistry Commons Recommended Citation Lu, Dongmei, "Synthesis And Characterization Of Metal Complexes Containing Tetrazolate, Poly(tetrazolyl)borate, And Aryl Pentazole Ligands As High Energy Density Materials" (2010). Wayne State University Dissertations. Paper 68. This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. SYNTHESIS AND CHARACTERIZATION OF METAL COMPLEXES CONTAINING TETRAZOLATE, POLY(TETRAZOLYL)BORATE, AND ARYL PENTAZOLE LIGANDS AS HIGH ENERGY DENSITY MATERIALS by DONGMEI LU DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2010 MAJOR: CHEMISTRY (Inorganic) Approved by: ______________________________ Advisor Date ______________________________ ______________________________ ______________________________ DEDICATION To my parents ii ACKNOWLEDGMENTS I would like to express my sincere gratitude to my advisor, Professor Charles H. Winter, for his guidance and support though the years at Wayne State University. I am grateful to my committee members, Professor Stephanie L. Brock, Professor Jin K. Cha, and Professor Mark Ming-Cheng Cheng, for reviewing my thesis and giving valuable comments and suggestions. I would like to thank Dr. Mary Jane Heeg for determining all the crystal structures, and Dr. Bashar Ksebati for his help with the NMR experiments. -

Forensic Analysis of Explosives
Forensic analysis of explosives Youngeun Choi, Dario Remmler, Maximilian Ries, Felix Rösicke, Radwan Sarhan, Felix Stete, Zhiyang Zhang Detecting and identifying explosives is of great importance ●Airport and airline security ●Demining Picture: Wo st 01/Wikipedia ●Removal of unexploded ordnance Picture: MatthiasKabel/Wikipedia ●Forensic analysis Picture: Tom Oates/Wikipedia Picture: Mark A. Moore/Wikipedia Outline ● Forensic analysis ● Common explosives ■ Inorganic explosives ● examples and sample preparation ● selected analytical techniques ■ Organic explosives containing Nitro-moieties ● Principle of detection ● selected analytical techniques ■ Other important explosives Forensic analysis After an incident with an explosion: Where was the source of the explosion? Which explosive was used? Where did the explosive come from? Commonly used explosives Inorganic explosives: Explosives with containing Nitro- Others: moieties: K++ + S + C Black powder Trinitrotoluene (TNT) Triacetone triperoxide (TATP) Dust of flammable materials Ammonium nitrate Nitroglycerin (NG) Inorganic Explosives Pure compounds Type Decomposition mechanism Characteristic ions − + Ammonium nitrate 2 NH4NO3 → 4 H2O + 2 N2 + O2 NO3 , NH4 − - + Ammonium perchlorate 2 NH4ClO4 → Cl2 + 2 O2 + N2 + 4 H2O NO3 , ClO4 , NH4 Ignition needed! Inorganic Explosives Pure compounds Mixtures Type Composition Characteristic ions − + + ANFO NH4NO3, fuel oil NO3 , NH4 , MeNH3 (Ammonium nitrate fuel oil) (long chain hydrocarbons) − 2- 2− Black powder Nitrates, sulfur, charcoal NO3 , SO4 , -

Enhanced Performance from Insensitive Explosives
Calhoun: The NPS Institutional Archive Faculty and Researcher Publications Faculty and Researcher Publications Collection 2013 Enhanced performance from insensitive explosives Brown, Ronald Monterey, California. Naval Postgraduate School 2013 Insensitive Munitions and Energetic Materials Technology SymposiumPaper 16169 http://hdl.handle.net/10945/47519 Approved for Public Release ENHANCED PERFORMANCE FROM INSENSITIVE EXPLOSIVES Ronald Brown, John Gamble, Dave Amondson, Ronald Williams, Paul Murch, and Joshua Lusk Physics Department Naval Postgraduate School, Monterey, CA 93943 Contact: [email protected] 2013 Insensitive Munitions and Energetic Materials Technology Symposium Paper 16169 Approved for Public Release Acknowledgement Dr. Kevin Vandersall Lawrence Livermore National Laboratory Technical Staff ANSYS-AUTODYN Berkeley, CA 2013 Insensitive Munitions and Energetic Materials Technology Symposium Paper 16169 Approved for Public Release Projected 14 Demonstrated Levels of Increase 12 “ONC” = Octanitrocubane “N8” = Octaazacubane > - e Overview of s c a e 10 e s r / c > - Achievements n m I e k s , a y e t i Relative to 8 r c c o n l I e V n o 6 i t Explosives a n o t Chronology e D 4 2 0 TNT RDX HMX CL-20 ONC N8 IMX HPX HPX+ 2013 Insensitive Munitions and Energetic Materials Technology Symposium Paper 16169 Approved for Public Release OUTLINE • Objective • Background • Modeling & Validations • Effect of Detonation Convergence on Energy Partitioning • Coaxial Initiation Limitations • Results of Novel Dynamic Compression • Conclusions 2013 Insensitive Munitions and Energetic Materials Technology Symposium Paper 16169 Approved for Public Release Develop means for enhancing directed energy from explosive weapon systems by exploiting the effects of overdriven detonation. Explore means for overcoming the limitations of coaxial charges. -

Local Minima Then Are Used As Starting Points for the Third Step of the Procedure
LA-I142-MSI CiC-14REPO”RTCollection -- c. 3 REPRODUCTION ‘“” COPY ~ -.;,. .1.! Los Alamos Nal,onal Laboratory IS operated by the Unwersity of Cal,fornla for the Umted States Department of Energy under conlracl W.7405.ENG.36. 1 -——.Procedqre for Estima ting the Crystal SN- ,-:~.-.....—.-:”.–:,--- ~~~=m --:.”-”..... ... Densities of Organzc ~xploswes ““”P. ~-. -. .. ...- .. ‘= - - -- .- — . Los Alamos National Laboratory ~O~~l~~~~LOSA,amOSN.WM~x,Co,,,,, 9 This work was supported by the U.S. Department of Energyand the Naval Surface Weapons Center, Silver Spring, Maryland. DISCLAIMER This repon waspreparedasanaeeount of worksponsoredbyan ageneyofthe UnitedStatesGovernment. Neithcrthe UnitedStatesGovernment noranyagcney thereof,noranyoftheiremployecs, makesany warmnty,expressor implied,or assumesany legalliabilityor responsibilityfor theaccuracy, completeness, or usefulnessof any information,apparatus.product, or prmessdiselosed,or representsthat its usewould not infringeprivatelyownedrighls.Referencehereinto any specificcommercial product, process,or service bytradersame, trademark,manufacturer,or otherwise,does not necessarilyconstituteor implyits endorsemermrecommendation,or favoringby~heUnitedStatesGovemmenl or any agencythereof.The viewsandopinionsof authorsexpressedhereindo not necessarilystateor reflect!hoseof the Uni~edStates Govemmermorany ageniy thereof. LA-11142-MS UC-45 Issued: November 1987 A Procedure for Estimatingthe Crystal Densities of Organic Explosives Don T. Cromer Herman L. Ammon’ James R. Holden** . -- , “. .—.-—. ,..—,.-- -
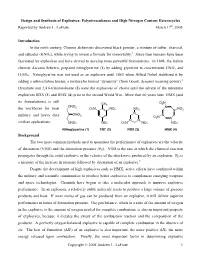
Design and Synthesis of Explosives: Polynitrocubanes and High Nitrogen Content Heterocycles Reported by Andrew L
Design and Synthesis of Explosives: Polynitrocubanes and High Nitrogen Content Heterocycles Reported by Andrew L. LaFrate March 17th, 2005 Introduction In the ninth century, Chinese alchemists discovered black powder, a mixture of sulfur, charcoal, 1 and saltpeter (KNO3), while trying to invent a formula for immortality. Since then humans have been fascinated by explosives and have strived to develop more powerful formulations. In 1846, the Italian chemist Ascanio Sobrero, prepared nitroglycerine (1) by adding glycerine to concentrated HNO3 and H2SO4. Nitroglycerine was not used as an explosive until 1863 when Alfred Nobel stabilized it by adding a nitrocellulose binder, a mixture he termed “dynamite” (from Greek: dynamis meaning power).2 Dynamite and 2,4,6-trinitrotoluene (2) were the explosives of choice until the advent of the nitramine explosives RDX (3) and HMX (4) prior to the second World War. More than 60 years later, HMX (and its formulations) is still O N CH3 2 ONO the workhorse for most 2 O N NO NO N NO 2 2 2 N 2 N military and heavy duty ONO2 N O2N N N N civilian applications. ONO2 NO2 O2N NO2 NO2 Nitroglycerine (1) TNT (2) RDX (3) HMX (4) Background The two most common methods used to quantitate the performance of explosives are the velocity of detonation (VOD) and the detonation pressure (PD). VOD is the rate at which the chemical reaction propagates through the solid explosive or the velocity of the shockwave produced by an explosion. PD is a measure of the increase in pressure followed by detonation of an explosive.3 Despite the development of high explosives such as HMX, active efforts have continued within the military and scientific communities to produce better explosives to complement emerging weapons and space technologies. -

Review of Some Newly Synthesized High Energetic Materials
Sci. Tech. Energetic Materials, Vol.65, No.6, 2004 215 Review Review of some newly synthesized high energetic materials S. Thangadurai*, K.P.S. Kartha**, D. R. Sharma**, and S. K. Shukla** *Department of Geology and Mining, Guindy, Chennai-600 032, INDIA e-mail: [email protected] (or) [email protected] **Central Forensic Science Laboratory, “Directorate of Forensic Sciences,” Govt. of India, Hyderabad-500 013, INDIA Received: April 12, 2004 Accepted: September 1, 2004 Abstract Towards the end of the last millennium many new high energetic materials were developed. These materials may replace the presently used explosives, sooner or later. In this review, the authors try to explore some of these materials viz., polynitropolycyclic cage explosives, cyclic nitramines, cage explosives, nitro derivatized heterocyclic compounds, nickel hydrazine nitrate (NHN) complex, nitrocubanes, hafnium explosives, heat-resistant explosives, new insensitive high explosives and some other novel high energetic materials. There is a need for the development of analytical methods for identification of these materials and their post explosion residues as these are likely to be encountered in crimes. 1. Introduction much as 80% ammonium nitrate. By the beginning of During the nineteenth century the developing science of World War II, the research to discover alternative explo- chemistry began to create molecular species with explosive sives resulted in another group of explosive molecules that properties. These molecules contain not only atoms that act could be used for the filling of ordnance 2). After two major as fuels, i.e. carbon and hydrogen, but also contain nitro World Wars in twentieth century, picric acid, TNT, tetryl, groups (NO2) similar to nitrates. -

High Explosive Mate...Pdf
Basic Characteristics for Estimation Polynitrogen Compounds Efficiency 233 Central European Journal of Energetic Materials, 2011, 8(4), 233-247 ISSN 1733-7178 Basic Characteristics for Estimation Polynitrogen Compounds Efficiency Alexandr SMIRNOV1, David LEMPERT2, Tatyana PIVINA3* and Dmitriy KHAKIMOV3 1 State Scientific Research Institute of Mechanical Engineering after V.V. Bakhirev, 11a Sverdlov St., Dzerzhinsk, Nizhny Novgorod Region, 606002, Russia 2 Institute of Problems of Chemical Physics, Russian Academy of Sciences, 1 Semenov Av., Chernogolovka, Moscow Region, 142432, Russia 3 Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky Prospect 47, Moscow 119991, Russia *E-mail: [email protected] Abstract: Basing on analysis of experimental data for physicochemical parameters of 300 energetic compounds from different chemical classes, a set of basic characteristics (enthalpies of formation, molecular crystals densities, detonation parameters, as well as sensitivity to different kinds of impact) has been considered and some calculations schemes have been elaborated to estimate these characteristics for polynitrogen compounds. The limits of physicochemical values for energetic materials (EMs) parameters have been estimated. A large set of compounds has been investigated and some of them seem to be rather effective as explosives or monopropellants. There are about ten powerful compounds (e.g. octanitrocubane, hexanitrohexaazaisowurtzitane, azanitrofurazan, etc.), explosives- oxidizers (such as ammonium dinitroamide and bis(difluroamine-dini troethil) nitroamine) and several hypothetic substances (not synthesized yet), such as octaazacubane, octaazatetraen, tetranitrotetraazacubane etc. Energetic properties were estimated as for individual compounds, as well as for energetic compositions containing these substances together with other ones in the aim to estimate the effectiveness of their use as components of solid composite propellants. -

A Theoretical Investigation of the Ring Strain Energy, Destabilization Energy, and Heat of Formation of CL-20
Hindawi Publishing Corporation Advances in Physical Chemistry Volume 2012, Article ID 175146, 7 pages doi:10.1155/2012/175146 Research Article A Theoretical Investigation of the Ring Strain Energy, Destabilization Energy, and Heat of Formation of CL-20 John A. Bumpus Department of Chemistry and Biochemistry, University of Northern Iowa, Cedar Falls, IA 50614, USA Correspondence should be addressed to John A. Bumpus, [email protected] Received 10 April 2012; Accepted 13 August 2012 Academic Editor: Dennis Salahub Copyright © 2012 John A. Bumpus. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The cage compound CL-20 (a.k.a., 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane, HNIW, or 2,4,6,8,10,12-hexanitro- 2,4,6,8,10,12-hexaazatetracyclo[5.5.0.03,11.05,9]dodecane) is a well-studied high-energy-density material (HEDM). The high Δ ◦ Δ ◦ positive gas- ( f Hg ) and solid- ( f Hs ) phase heat of formation values for CL-20 conformers have often been attributed to the strain energy of this cage compound and, by implication, to the conventional ring strain energy (CRSE) inherent in isowurtzitane Δ ◦ which may be viewed as a “parent compound” (although not the synthetic precursor) of CL-20. f Hg values and destabilization energies (DSEs), which include the contribution from CRSE, were determined by computation using a relatively new multilevel ab intio model chemistry. Compared to cubane, isowurtzitane does not have an exceptionally high CRSE. -
![Hepta- and Octanitrocubanes** Mocubenes, Cubene, Cubyl Carbinyl Radical, Cubyl Cation, Cubanediyl, and the [N]Cubyls.[6] Mao-Xi Zhang, Philip E](https://docslib.b-cdn.net/cover/3097/hepta-and-octanitrocubanes-mocubenes-cubene-cubyl-carbinyl-radical-cubyl-cation-cubanediyl-and-the-n-cubyls-6-mao-xi-zhang-philip-e-4643097.webp)
Hepta- and Octanitrocubanes** Mocubenes, Cubene, Cubyl Carbinyl Radical, Cubyl Cation, Cubanediyl, and the [N]Cubyls.[6] Mao-Xi Zhang, Philip E
COMMUNICATIONS Hepta- and Octanitrocubanes** mocubenes, cubene, cubyl carbinyl radical, cubyl cation, cubanediyl, and the [n]cubyls.[6] Mao-Xi Zhang, Philip E. Eaton,* and Richard Gilardi As we have reported previously,[7] 1,3,5,7-tetranitrocubane (1) can be made by dimethyldioxirane oxidation of the Highly nitrated cubanes are predicted to be shock-insensi- tetraamine derived from Curtius-type transformations of the tive, very dense, high-energy compounds with great potential corresponding tetraacid, itself made either from ortho-metal- as explosives and propellants.[1] Application of the Kamlett ± ation of amide-activated cubanes[7a] or from photochlorocar- Jacobs equations[2] to octanitrocubane using predicted values bonylation of cubane monoacid.[7b, 8] More highly nitrated for density (1.9 ± 2.2 gcm3)[3] and DH (81 ± 144 kcal mol1)[4] f cubanes cannot be made similarly from more highly carboxy- leads to calculated detonation velocities and pressures much lated cubanes, as the conversion would proceed through higher than that of the classic C-nitro explosive TNT, 15 ± intermediates that contain an electron-donating group vicinal 30% greater than that of the N-nitro compound HMX, to a group that is electron-withdrawing; this situation results presently the most energetic of standard military explosives, unavoidably in cleavage of the highly strained cubane cage.[7b] and perhaps even better than that of the experimental As shown earlier,[7b, 9] 1 is quite acidic (pK 21). Nitration polycyclic nitramine CL-20, arguably the most powerful a of its anion (e.g., as the sodium salt) at the melting (approx. nonnuclear explosive known.[5] 1058C) interface between frozen THF and N2O4 gave 1,2,3,5,7-pentanitrocubane (2), the first nitrocubane with NO2 CH3 NO2 [10] NO2 adjacent nitro groups. -

VB 2019 HEM No Layer
High Energy Materials O2N NO2 N N N N N3 N N N NO2 NO O2N 2 NO O2N 2 NO O2N 2 O2N NO2 Vlad Bacauanu MacMillan Research Group Group Meeting July 25th, 2019 What Are High Energy Materials? compound that stores chemical energy high energy material and which upon stimulation undergoes (HEM) rapid decomposition with release of energy and gas explosives propellants pyrotechnics Applications of High Energy Materials The obvious – military applications Civil and scientific applications mining civil engineering oil industry space exploration High Energy Materials – Outline Generals aspects of high energy materials Very brief history Fundamental properties Decomposition via deflagration or detonation Primary vs secondary explosives Oxygen balance Synthesis of high energy materials Safety precautions in HEM labs Explosophore functional groups Examples of synthetic approaches Recent avenues within the field Nitrogen-rich molecules Strained energetic materials: HNIW and ONC Exploring stereochemistry of materials Book References for High Energy Materials High Energy Materials Chemistry of High-Energy Materials Organic Chemistry of Explosives Jai P. Agrawal Thomas M. Klapötke Jai P. Agrawal & Robert D. Hodgson 1st ed. (2010) 4th ed. (2017) 1st ed. (2007) History of Development of High Energy Materials 1943 CH3 O2N NO2 gunpowder N N O2N NO2 (KNO3 + C + S) N N 1863 1880 TNT O NO 1894 O2N NO2 2 HMX ONO2 NO2 O2NO ONO2 O2NO ONO2 PETN nitroglycerin ONO2 800 1000 1850 1900 1950 present OH O N NO 2 N N 2 1855 O2N NO2 O N 2 N N NO2 ONO 2 picric O2NO 1885 1930’s N N O acid O N NO O NO 2 CL-20 2 2 O2N NO2 N N ONO 1987 2 n RDX nitrocellulose 1867 N dynamite NO2 in Radical Chemistry, 1st ed.;, C., Wiley-VCH: Weinheim, Germany, 2004.