Cyanogenesis in the Euphorbiaceae Lucinda L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Revision and Phylogeny of Acalypha (Euphorbiaceae) in Malesia
Blumea 55, 2010: 21–60 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE doi:10.3767/000651910X499141 Revision and phylogeny of Acalypha (Euphorbiaceae) in Malesia V.G. Sagun1,2, G.A. Levin2, P.C. van Welzen3 Key words Abstract Twenty-eight species of Acalypha are recognized in Malesia. Acalypha paniculata is the sole member of subgenus Linostachys in Malesia and the rest of the species belong to subgenus Acalypha. Four previously Acalypha synonymized species are resurrected as distinct species, namely A. angatensis, A. cardiophylla var. cardiophylla, Euphorbiaceae A. grandis, and A. wilkesiana. Four species names are newly reduced to synonymy. The molecular phylogenetic Malesia analyses indicate that Acalypha is monophyletic, as is the subgenus Acalypha. The early-diverging lineages in the phylogeny genus, and its closest outgroup, consist of African species. The Malesian species do not form a monophyletic group although the molecular data strongly support two small clades within the region that are morphologically homogene- ous. The classification system that Pax and Hoffmann applied to subgenus Acalypha, which is based primarily on inflorescence morphology, appears to be unsatisfactory and incongruent with the phylogenetic analyses. Published on 16 April 2010 INTRODUCTION Molecular systematics confirms the placement of Acalypha in Acalyphoideae s.s. and shows a close relationship between Acalypha L. is the third largest genus in the Euphorbiaceae Acalypha and Mareya Baill. (Wurdack et al. 2005, Tokuoka s.s. after Euphorbia L., and Croton L., having about 450 spe- 2007). Their relationship is supported by similar morphologi- cies worldwide (Webster 1994, Radcliffe-Smith 2001). In the cal characteristics, including laciniate styles, pendulous anther Malesian region, 28 species of Acalypha are recognized herein. -

CHAPTER 2 REVIEW of the LITERATURE 2.1 Taxa And
CHAPTER 2 REVIEW OF THE LITERATURE 2.1 Taxa and Classification of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.11 Taxa and Classification of Acalypha indica Linn. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Euphorbiales Family : Euphorbiaceae Subfamily : Acalyphoideae Genus : Acalypha Species : Acalypha indica Linn. (Saha and Ahmed, 2011) Plant Synonyms: Acalypha ciliata Wall., A. canescens Wall., A. spicata Forsk. (35) Common names: Brennkraut (German), alcalifa (Brazil) and Ricinela (Spanish) (36). 9 2.12 Taxa and Classification of Bridelia retusa (L.) A. Juss. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Bridelia Species : Bridelia retusa (L.) A. Juss. Plant Synonyms: Bridelia airy-shawii Li. Common names: Ekdania (37,38). 2.13 Taxa and Classification of Cleidion javanicum BL. Kingdom : Plantae Subkingdom : Tracheobionta Superdivision : Spermatophyta Division : Magnoliophyta Class : Magnoliopsida Subclass : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Cleidion Species : Cleidion javanicum BL. Plant Synonyms: Acalypha spiciflora Burm. f. , Lasiostylis salicifolia Presl. Cleidion spiciflorum (Burm.f.) Merr. Common names: Malayalam and Yellari (39). 10 2.2 Review of chemical composition and bioactivities of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.2.1 Review of chemical composition and bioactivities of Acalypha indica Linn. Acalypha indica -
![Entry for ACALYPHA Acrogyna Pax [Family EUPHORBIACEAE]](https://docslib.b-cdn.net/cover/8673/entry-for-acalypha-acrogyna-pax-family-euphorbiaceae-78673.webp)
Entry for ACALYPHA Acrogyna Pax [Family EUPHORBIACEAE]
Entry for ACALYPHA acrogyna Pax [family EUPHORBIACEAE] http://plants.jstor.org/flora/flota011327 http://www.jstor.org Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the contributing partner regarding any further use of this work. Partner contact information may be obtained at http://plants.jstor.org/page/about/plants/PlantsProject.jsp. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Page 1 of 2 Entry for ACALYPHA acrogyna Pax [family EUPHORBIACEAE] Herbarium Royal Botanic Gardens, Kew (K) Collection Flora of Tropical Africa Resource Type Reference Sources Entry from Flora of Tropical Africa, Vol 6 Part 1, page 441 (1913) Author: (By J. G. Baker, with additions by C. H. Wright.) Names ACALYPHA acrogyna Pax [family EUPHORBIACEAE], in Engl. Jahrb. -
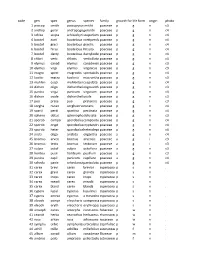
Species List (PDF)
code gen spec genus species family growth formlife form origin photo 1 pascop smith pascopyrumsmithii poaceae p g n c3 2 androp gerar andropogongerardii poaceae p g n c4 3 schiza scopa schizachyriumscoparium poaceae p g n c4 4 boutel curti bouteloua curtipendulapoaceae p g n c4 5 boutel graci bouteloua gracilis poaceae p g n c4 6 boutel hirsu bouteloua hirsuta poaceae p g n c4 7 boutel dacty bouteloua dactyloidespoaceae p g n c4 8 chlori verti chloris verticillata poaceae p g n c4 9 elymus canad elymus canadensispoaceae p g n c3 10 elymus virgi elymus virginicus poaceae p g n c3 11 eragro spect eragrostis spectabilis poaceae p g n c4 12 koeler macra koeleria macrantha poaceae p g n c3 13 muhlen cuspi muhlenbergiacuspidata poaceae p g n c4 14 dichan oligo dichantheliumoligosanthespoaceae p g n c3 15 panicu virga panicum virgatum poaceae p g n c4 16 dichan ovale dichantheliumovale poaceae p g n c3 17 poa prate poa pratensis poaceae p g i c3 18 sorgha nutan sorghastrumnutans poaceae p g n c4 19 sparti pecti spartina pectinata poaceae p g n c4 20 spheno obtus sphenopholisobtusata poaceae p g n c3 21 sporob compo sporoboluscomposituspoaceae p g n c4 22 sporob crypt sporoboluscryptandruspoaceae p g n c4 23 sporob heter sporobolusheterolepispoaceae p g n c4 24 aristi oliga aristida oligantha poaceae a g n c4 25 bromus arven bromus arvensis poaceae a g i c3 26 bromus tecto bromus tectorum poaceae a g i c3 27 vulpia octof vulpia octoflora poaceae a g n c3 28 hordeu pusil hordeum pusillum poaceae a g n c3 29 panicu capil panicum capillare poaceae a g n c4 30 schedo panic schedonnarduspaniculatuspoaceae p g n c4 31 carex brevi carex brevior cyperaceaep s n . -

Species Composition, Diversity, and Stand Structure of Tropical Lower Montane Forests Resulting from Various Human Impacts on the Shan Plateau, Eastern Myanmar
ISSN : 0917-415X DOI:10.3759/tropics.MS17-03 TROPICS Vol. 26 (3) 71-82 Issued December 1, 2017 ORIGINAL ARTICLE Species composition, diversity, and stand structure of tropical lower montane forests resulting from various human impacts on the Shan Plateau, eastern Myanmar Phyu Phyu Lwin1, 2* and Mamoru Kanzaki1 1 Graduate School of Agriculture, Kyoto University, Kyoto 606‒8502, Japan 2 Present address: Forest Research Institute, Forest Department, Ministry of Natural Resources and Environmental Conservation, Myanmar * Corresponding author: [email protected] Received: May 30, 2017 Accepted: October 2, 2017 ABSTRACT We observed species composition, diversity, and stand structure in the tropical lower montane forests of a hilly region in eastern Myanmar and examined the impacts of anthropogenic disturbances on the forest stands. From our survey of 58 sample plots (30 m×30 m), we categorized four stand types using nonmetric multi-dimensional scaling (NMS) ordination. The four stand types exhibited significant contributions of various anthropogenic impacts, reflecting differences in local livelihoods within different varying landscapes. Anthropogenic disturbances, especially the extraction of firewood, can significantly affect the stand structure of forests and, in turn, the species composition and tree diversity. Some early successional species such as Phyllanthus albizzioides and Albizia odoratissima became indicator species of highly disturbed forests. As firewood is mainly extracted from privately owned forests rather than communal forests, land tenure was also an important factor governing the intensity of anthropogenic disturbances. Species richness and diversity values decreased in stand types exposed to more severe anthropogenic disturbances. Stem density was significantly higher in highly disturbed forests. This was a result of higher numbers of multi-stemmed individuals, which revealed the effect of cutting larger stems for firewood extraction. -

Czech University of Life Sciences Prague
Czech University of Life Sciences Prague Faculty of Tropical AgriSciences Molecular Characterization of Plukenetia volubilis L. and Analysis of Seed Storage Protein Pattern and Protein Fractions Dissertation Thesis Department of Crop Sciences and Agroforestry Author: Ing. Martin Ocelák Supervisor: doc. Ing. Bohdan Lojka, Ph.D. Co-supervisors: Ing. Petra Hlásná Čepková, Ph.D. Ing. Iva Viehmannová, Ph.D. In Prague, September, 2016 Acknowledgment I would like to express my gratitude to my supervisor doc. Ing. Bohdan Lojka, Ph.D. and co-supervisors Ing. Petra Hlásná Čepková, Ph.D. and Ing. Iva Viehmannová, Ph.D. for their guidance, advices, help and also patience during the studies, laboratory works and mainly during the writings. My thanks also belong to IIAP represented by Ing. Danter Cachique Huansi and Lucas Garcia Chujutalli for their cooperation in samples collection, to Ing. Anna Prohasková for her guidance during analysis of proteins in Crop Research Institute in Prague - Ruzyně, to Ing. Eva Beoni, Ph.D. and Ing. Lenka Havlíčková, Ph.D. for their help in learning how to work in the laboratory; to Ing. Zdislava Dvořáková, Ph.D. for her help, teaching and encouragement and to Ing. Blanka Křivánková, Ph.D. for providing some useful materials. Also my family contributed with their support in all means. So great thanks belong to my parents Jan and Jaroslava Ocelákovi and my boyfriend Ioannis Nikolakis for their love and support in all possible means. This research was supported financially by an Internal Grant Agency of the University of Life Science Prague, CIGA (Project No. 20135004), by an Internal Grant Agency of the Faculty of Tropical AgriSciences - University of Life Science Prague, IGA (Project No. -

Survey of Euphorbiaceae Family in Kopergaon Tehsil Of
International Journal of Humanities and Social Sciences (IJHSS) ISSN (P): 2319–393X; ISSN (E): 2319–3948 Vol. 9, Issue 3, Apr–May 2020; 47–58 © IASET SURVEY OF EUPHORBIACEAE FAMILY IN KOPERGAONTEHSIL OF MAHARASHTRA Rahul Chine 1 & MukulBarwant 2 1Research Scholar, Department of Botany, Shri Sadguru Gangagir Maharaj Science College, Maharashtra, India 2Research Scholar, Department of Botany, Sanjivani Arts Commerce and Science College, Maharashtra, India ABSTRACT The survey of Family Euphorbiaceae from Kopargoantehshil is done. In this we first collection of different member of Family Euphorbiaceae from different region of Kopargoantehasil. An extensive and intensive survey at plants was carried out from village Pathare, Derde, Pohegoan, Kopergaon, Padhegaon, Apegoan during the were collected in flowering and fruiting period throughout the year done. During survey we determine 16 member of Euphorbiceae from Kopargoantehshil Then we decide characterization on the basis of habit, flowering character, leaf and fruit character with help of that character and using different literature we identified each and every member of Euphorbiaceae Species were identified with relevant information and documented in this paper with regard to their Botanical Name, family, Habitat, flowering Fruiting session and their medicinal value of some member of Euphorbiaceae that used in medicine respiratory disorder such as cough, asthama, bronchitis etc and some are toxic in nature due to their toxic latex that is showing itching reaction. KEYWORDS : Family Euphorbiaceae, Respiratory Ailment, Identification, Chracterization and Documentation Article History Received: 09 Apr 2020 | Revised: 10 Apr 2020 | Accepted: 18 Apr 2020 INTRODUCTION The Euphorbiaceae, the spurge family, is one of the complex large family of flowering plants of angiosperm with 334 genera and 8000 species in the worlds (Wurdack 2004). -

Aleurites Fordii Hemsl.) (Euphorbiaceae): New to the Arkansas Flora Brett Es Rviss Henderson State University, [email protected]
Journal of the Arkansas Academy of Science Volume 61 Article 24 2007 Tungoil Tree (Aleurites fordii Hemsl.) (Euphorbiaceae): New to the Arkansas Flora Brett eS rviss Henderson State University, [email protected] Nicole Freeman Henderson State University Joslyn Hernandez Henderson State University Allen Leible Henderson State University Chris Talley Henderson State University Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Plant Biology Commons Recommended Citation Serviss, Brett; Freeman, Nicole; Hernandez, Joslyn; Leible, Allen; and Talley, Chris (2007) "Tungoil Tree (Aleurites fordii Hemsl.) (Euphorbiaceae): New to the Arkansas Flora," Journal of the Arkansas Academy of Science: Vol. 61 , Article 24. Available at: http://scholarworks.uark.edu/jaas/vol61/iss1/24 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This General Note is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected]. - Journal of the Arkansas Academy of Science, Vol. 61 [2007], Art. 24 Tungoil Tree (Alellritesfordii Hemsl.) (Euphorbiaceae) New to the Arkansas Flora !Henderson State University, Biology Department, P.O Box H-7570, Arkadelphia, AR 71999-0001 ICorrespondence: [email protected] The problems associated with the introduction, subsequent and become invasive in Arkansas and elsewhere in the United establishment, and naturalization ofnon-native plant species in States following intentional introduction. -

National Parks and Wildlife Act 1972.PDF
Version: 1.7.2015 South Australia National Parks and Wildlife Act 1972 An Act to provide for the establishment and management of reserves for public benefit and enjoyment; to provide for the conservation of wildlife in a natural environment; and for other purposes. Contents Part 1—Preliminary 1 Short title 5 Interpretation Part 2—Administration Division 1—General administrative powers 6 Constitution of Minister as a corporation sole 9 Power of acquisition 10 Research and investigations 11 Wildlife Conservation Fund 12 Delegation 13 Information to be included in annual report 14 Minister not to administer this Act Division 2—The Parks and Wilderness Council 15 Establishment and membership of Council 16 Terms and conditions of membership 17 Remuneration 18 Vacancies or defects in appointment of members 19 Direction and control of Minister 19A Proceedings of Council 19B Conflict of interest under Public Sector (Honesty and Accountability) Act 19C Functions of Council 19D Annual report Division 3—Appointment and powers of wardens 20 Appointment of wardens 21 Assistance to warden 22 Powers of wardens 23 Forfeiture 24 Hindering of wardens etc 24A Offences by wardens etc 25 Power of arrest 26 False representation [3.7.2015] This version is not published under the Legislation Revision and Publication Act 2002 1 National Parks and Wildlife Act 1972—1.7.2015 Contents Part 3—Reserves and sanctuaries Division 1—National parks 27 Constitution of national parks by statute 28 Constitution of national parks by proclamation 28A Certain co-managed national -

Kalbarri National Park ‘Nature’S Window’
Kalbarri National Park ‘nature’s window’ draft management plan 2014 Department of Parks and Wildlife Locked Bag 104 Bentley Delivery Centre WA 6983 Phone: (08) 9219 9000 Fax: (08) 9334 0498 www.dpaw.wa.gov.au © State of Western Australia 2014 April 2014 This work is copyright. You may download, display, print and reproduce this material in unaltered form (retaining this notice) for your personal, non-commercial use or use within your organisation. Apart from any use as permitted under the Copyright Act 1968, all other rights are reserved. Requests and enquiries concerning reproduction and rights should be addressed to the Department of Parks and Wildlife. ISBN 978-1-921703-47-8 (print) ISBN 978-1-921703-48-5 (online) This draft management plan was prepared by the Conservation Commission of Western Australia through the agency of the Department of Parks and Wildlife. Questions regarding the use of this material should be directed to: Planning Branch Department of Parks and Wildlife 17 Dick Perry Avenue, Kensington WA 6151 Locked Bag 104 Bentley Delivery Centre WA 6983 Phone: (08) 9219 9000 Email: [email protected] The recommended reference for this publication is: Department of Parks and Wildlife 2014, Kalbarri National Park draft management plan 2014, Department of Parks and Wildlife, Perth. This document is available in alternative formats on request. Please note: URLs in this document which conclude a sentence are followed by a full point. If copying the URL please do not include the full point. Front cover photos Main Natures Window at The Loop. Photo – Melissa Mazzella (DPaW) Top left Red kangaroo. -

Ethnobotanical Observations of Euphorbiaceae Species from Vidarbha Region, Maharashtra, India
Ethnobotanical Leaflets 14: 674-80, 2010. Ethnobotanical Observations of Euphorbiaceae Species from Vidarbha region, Maharashtra, India G. Phani Kumar* and Alka Chaturvedi# Defence Institute of High Altitude Research (DRDO), Leh-Ladakh, India #PGTD Botany, RTM Nagpur University, Nagpur, India *corresponding author: [email protected] Issued: 01 June, 2010 Abstract An attempt has been made to explore traditional medicinal knowledge of plant materials belonging to various genera of the Euphorbiaceae, readily available in Vidharbha region of Maharasthtra state. Ethnobotanical information were gathered through several visits, group discussions and cross checked with local medicine men. The study identified 7 species to cure skin diseases (such as itches, scabies); 5 species for antiseptic (including antibacterial); 4 species for diarrhoea; 3 species for dysentery, urinary infections, snake-bite and inflammations; 2 species for bone fracture/ dislocation, hair related problems, warts, fish poisons, night blindness, wounds/cuts/ burns, rheumatism, diabetes, jaundice, vomiting and insecticide; 1 species as laxative , viral fever and arthritis. The results are encouraging but thorough scientific scrutiny is absolutely necessary before being put into practice. Key words: Ethnopharmacology; Vidarbha region; Euphorbiaceae; ethnobotanical information. Introduction The medicinal properties of a plant are due to the presence of certain chemical constituents. These chemical constituents, responsible for the specific physiological action, in the plant, have in many cases been isolated, purified and identified as definite chemical compounds. Quite a large number of plants are known to be of medicinal use remain uninvestigated and this is particularly the case with the Indian flora. The use of plants in curing and healing is as old as man himself (Hedberg, 1987). -

9. Herbs and Its Amazing Healing Properties
EPTRI‐ENVIS Centre (Ecology of Eastern Ghats) HERBS AND ITS AMAZING HEALING PROPERTIES Article 04/2015/ENVIS-Ecology of Eastern Ghats Page 1 of 50 EPTRI‐ENVIS Centre (Ecology of Eastern Ghats) LIST OF MEDICINAL HERBS Plant name : Achyranthes aspera L. Family : Amaranthaceae Local name : Uttareni Habit : Herb Fl & Fr time : October – March Part(s) used : Leaves Medicinal uses : Leaf paste is applied externally for eye pain and dog bite. Internally taken leaves decoction with water/milk to cure stomach problems, diuretic, piles and skin diseases. Plant name : Abelmoschus esculentus (L.) Moench. Family : Malvaceae Local name : Benda Habit : Herb Fl & Fr time : Part(s) used : Roots Medicinal uses : The juice of the roots is used externally to treat cuts, wounds and boils. Plant name : Abutilon crispum (L.) Don Family : Malvaceae Local name : Nelabenda Habit : Herb Fl & Fr time : March – September Part(s) used : Root Medicinal uses : Root is used for the treatment of nervous disorders. Article 04/2015/ENVIS-Ecology of Eastern Ghats Page 2 of 50 EPTRI‐ENVIS Centre (Ecology of Eastern Ghats) Plant name : Abutilon indicum (L.) Sweet Family : Malvaceae Local name : Thuttutubenda Habit : Herb Fl & Fr time : March – September Part(s) used : Leaves & Roots Medicinal uses : Leaf juice is used for the treatment of toothache. Roots and leaves decoction is given for diuretic and stimulate purgative. Plant name : Abrus precatorius L. Family : Fabaceae Local name : Guruvenda Habit : Herb Fl & Fr time : July – December Part(s) used : Root & Seeds Medicinal uses : Roots used to treat poisonous bite and seed is used to treat leucoderma Plant name : Acalypha indica L.