Seamount Science
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (Ogrefish) by J.A
click for previous page 1178 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths (ogrefish) by J.A. Moore, Florida Atlantic University, USA iagnostic characters: Small (to about 160 mm standard length) beryciform fishes.Body short, deep, and Dcompressed, tapering to narrow peduncle. Head large (1/3 standard length). Eye smaller than snout length in adults, but larger than snout length in juveniles. Mouth very large and oblique, jaws extend be- hind eye in adults; 1 supramaxilla. Bands of villiform teeth in juveniles are replaced with large fangs on dentary and premaxilla in adults; vomer and palatines toothless. Deep sensory canals separated by ser- rated ridges; very large parietal and preopercular spines in juveniles of one species, all disappearing with age. Gill rakers as clusters of teeth on gill arch in adults (lath-like in juveniles). No true fin spines; single, long-based dorsal fin with 16 to 20 rays; anal fin very short-based with 7 to 9 soft rays; caudal fin emarginate; pectoral fins with 13 to 16 soft rays; pelvic fins with 7 soft rays. Scales small, non-overlapping, spinose, goblet-shaped in adults; lateral line an open groove partially bridged by scales; no enlarged ventral keel scutes. Colour: entirely dark brown or black in adults. Habitat, biology, and fisheries: Meso- to bathypelagic, at depths of 75 to 5 000 m. Carnivores, with juveniles feeding on mainly crustaceans and adults mainly on fishes. May sometimes swim in small groups. Uncommon deep-sea fishes of no commercial importance. Remarks: The family was revised recently by Kotlyar (1986) and contains 1 genus with 2 species throughout the tropical and temperate latitudes. -

Inferences on Potential Seamount Locations from Mid-Resolution
T. Morato and D. Pauly (eds.), Seamounts: Biodiversity and Fisheries, Page 7 INFERENCES ON POTENTIAL SEAMOUNT LOCATIONS FROM MID- RESOLUTION BATHYMETRIC DATA Adrian Kitchingman and Sherman Lai Fisheries Centre, The University of British Columbia. 2259 Lower Mall, Vancouver, B.C., V6T 1Z4, Canada [email protected]; [email protected] ABSTRACT Seamounts are underwater volcanoes that did not grow tall enough to break to the sea surface and turn into islands. Once formed, seamounts tend to gradually sink under their own weight and the subsidence of the lithosphere. The ocean floor is littered with these former seamounts, here called ‘seamounds’. Seamounts occur throughout the world's oceans, but their number (which may surpass 50,000) is difficult to estimate, even roughly, because it depends on the resolution of the bathymetric map used and the specific definition of a seamount used, i.e., the limits used to distinguish between seamounts and seamounds. Here, the locations of a subset of the seamounts of the world were identified using two algorithms relying on the depth differences between adjacent cells of a digital global elevation map distributed by the U.S. National Oceanographic and Atmospheric Agency (NOAA). The overlap of both algorithms resulted in a set of about 14,000 seamounts, but a different number would have been found had we used different thresholds. Known seamount locations supplied by NOAA and SeamountsOnline (http://seamounts.sdsc.edu) were compared against the corresponding seamounts located by the study, which led to some degree of ground-truthing. The coordinates of the seamounts identified in this study are available on the CD-ROM attached to this report, and on http://www.seaaroundus.org. -

Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA
ACTA ICHTHYOLOGICA ET PISCATORIA (2016) 46 (4): 287–291 DOI: 10.3750/AIP2016.46.4.02 MICROSATELLITE DNA-BASED GENETIC TRACEABILITY OF TWO POPULATIONS OF SPLENDID ALFONSINO, BERYX SPLENDENS (ACTINOPTERYGII: BERYCIFORMES: BERYCIDAE)—PROJECT CELFISH—PART 2 Jolanta KEMPTER*, Maciej KIEŁPIŃSKI, Remigiusz PANICZ, and Sławomir KESZKA Division of Aquaculture, West Pomeranian University of Technology, Szczecin, Kazimierza Krolewicza 4, 71-550 Szczecin, Poland Kempter J., Kiełpinski M., Panicz R., Keszka S. 2016. Microsatellite DNA-based genetic traceability of two populations of splendid alfonsino, Beryx splendens (Actinopterygii: Beryciformes: Berycidae)— Project CELFISH—Part 2. Acta Ichthyol. Piscat. 46 (4): 287–291. Background. The study is a contribution to Project CELFISH which involves genetic identifi cation of populations of fi sh species presenting a particular economic importance or having a potential to be used in the so-called commercial substitutions. The EU fi sh trade has been showing a distinct trend of more and more fi sh species previously unknown to consumers being placed on the market. Molecular assays have become the only way with which to verify the reliability of exporters. This paper is aimed at pinpointing genetic markers with which to label and differentiate between two populations of splendid alfonsino, Beryx splendens Lowe, 1834, a species highly attractive to consumers in Asia and Oceania due to the meat taste and low fat content. Material and methods. DNA was isolated from fragments of fi ns collected at local markets in Japan (MJ) (n = 10) and New Zealand (MNZ) (n = 18). The rhodopsin gene (RH1) fragment and 16 microsatellite DNA fragments (SSR) were analysed in all the individuals. -

Order BERYCIFORMES ANOPLOGASTRIDAE Anoplogaster
click for previous page 2210 Bony Fishes Order BERYCIFORMES ANOPLOGASTRIDAE Fangtooths by J.R. Paxton iagnostic characters: Small (to 16 cm) Dberyciform fishes, body short, deep, and compressed. Head large, steep; deep mu- cous cavities on top of head separated by serrated crests; very large temporal and pre- opercular spines and smaller orbital (frontal) spine in juveniles of one species, all disap- pearing with age. Eyes smaller than snout length in adults (but larger than snout length in juveniles). Mouth very large, jaws extending far behind eye in adults; one supramaxilla. Teeth as large fangs in pre- maxilla and dentary; vomer and palatine toothless. Gill rakers as gill teeth in adults (elongate, lath-like in juveniles). No fin spines; dorsal fin long based, roughly in middle of body, with 16 to 20 rays; anal fin short-based, far posterior, with 7 to 9 rays; pelvic fin abdominal in juveniles, becoming subthoracic with age, with 7 rays; pectoral fin with 13 to 16 rays. Scales small, non-overlap- ping, spinose, cup-shaped in adults; lateral line an open groove partly covered by scales. No light organs. Total vertebrae 25 to 28. Colour: brown-black in adults. Habitat, biology, and fisheries: Meso- and bathypelagic. Distinctive caulolepis juvenile stage, with greatly enlarged head spines in one species. Feeding mode as carnivores on crustaceans as juveniles and on fishes as adults. Rare deepsea fishes of no commercial importance. Remarks: One genus with 2 species throughout the world ocean in tropical and temperate latitudes. The family was revised by Kotlyar (1986). Similar families occurring in the area Diretmidae: No fangs, jaw teeth small, in bands; anal fin with 18 to 24 rays. -

How Much Longer Will It Take?
How much longer will it take? A ten-year review of the implementation of United Nations General Assembly resolutions 61/105, 64/72 and 66/68 on the management of bottom fisheries in areas beyond national jurisdiction FULL REPORT – AUGUST 2016 DAVID SHALE/NATURE PICTURE LIBRARAY SHALE/NATURE DAVID Leiopathes sp., a deepwater black coral, has lifespans in excess of 4,200 years (Roark et al., 2009*), making it one of the oldest living organism on Earth. Specimen was located off the coast of Oahu, Hawaii, in ~400 m water depth. * Roark, E.B., Guilderson, T.P., Dunbar, R.B., Fallon, S.J., and Mucciarone, D.A., 2009. Extreme longevity in proteinaceous deep-sea corals. Proceedings of the National Academy of Sciences, 106: 520– 5208, doi: 10.1073/pnas.0810875106. © HAWAII UNDERSEA RESEARCH LABORATORY, TERRY KERBY AND MAXIMILIAN CREMER Contents Executive summary 03 1.0 Introduction 09 2.0 North Atlantic 10 2.1 Northeast Atlantic 10 2..2 Northwest Atlantic 21 3.0 South Atlantic 33 3.1 Southeast Atlantic 33 3.2 Southwest Atlantic and other non-RFMO areas 39 4.0 North Pacific 42 Citation: 5.0 South Pacific 49 Gianni, M., Fuller, S.D., Currie, D.E.J., Schleit, 6.0 Indian Ocean 60 K., Goldsworthy, L., Pike, B., Weeber, B., 7.0 Southern Ocean 66 Owen, S., Friedman, A. How much longer will it take? A ten-year 8.0. Mediterranean Sea 71 review of the implementation of United Annex 1. Acronyms 73 Nations General Assembly resolutions Annex 2. History of the UNGA negotiations 73 61/105, 64/72 and 66/68 on the management Annex 3. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Importance of Seamount-Like Features for Conserving Mediterranean Marine Habitats and Threatened Species
Nº. 1105 IMPORTANCE OF SEAMOUNT-LIKE FEATURES FOR CONSERVING MEDITERRANEAN MARINE HABITATS AND THREATENED SPECIES Ricardo Aguilar, Xavier Pastor, Silvia García & Pilar Marín Oceana. Leganitos, 47 - 28013 Madrid. Spain - *[email protected] INTRODUCTION HABITAT LOCATION MAIN SPECIES 16 of the more than 200 seamount‑like peaks in the Mediterranean1 have been observed using ROV. Coral reefs 4, 5, 6, 7 Lophelia pertusa, Madrepora oculata Desmophyllum dianthus, Stenocyathus vermiformis, The findings, which include carnivorous sponges, elasmobranches, coral gardens, sponge aggregations, Cold water corals All sites Caryophyllia spp. Pourtalosmilia anthophyllites, Javania caileti, coralligenous beds, as well as species new to science like the giant foraminifera Spiculosiphon oceana, Anomocora fecunda, Dendrophyllia spp. Paramuricea spp., Eunicella spp., Viminella flagellum, or new to the Mediterranean, like the scleractinian Anomocora fecunda, underscore the importance of 1, 2, 3, 4, 5, Callogorgia verticillata, Acanthogorgia spp., Placogorgia these geological features as hotspots and shelters/refuges species and habitats that are threatened, in Gorgonian/coral gardens: 6, 7, 8, 9, 11, coronata, Swiftia pallida, Muriceides lepida, Villogorgia regression, or rare in other Mediterranean areas. 12, 13, 14 bebrycoides, Bebryce mollis, Nicella granifera 1, 4, 5, 6, 7, Leiopathes glaberrima, Antipathes dichotoma, Antipathella METHODOLOGY Black corals Since 2006, Oceana has carried out six expeditions in the Mediterranean performing 129 ROV’s dives 9, 11, 15, 16 subpinnata, Parantipathes larix Isidella elongata, Pennatula spp., Pteroeides griseum, 2, 3, 4, 7, 8, over 16 seamounts, between ‑37 and ‑638 meters deep. Soft bottoms’ octocorals Virgularia mirabilis, Veretillun cynomorium, Kophobelemnon 9, 11, 14, 16 RESULTS stelliferum, Funiculina quadrangularis 2 3, 4, 5, 6, 7, Asconema setubalense, Phakellia spp., Axinella spp. -

Chapter 51. Biological Communities on Seamounts and Other Submarine Features Potentially Threatened by Disturbance
Chapter 51. Biological Communities on Seamounts and Other Submarine Features Potentially Threatened by Disturbance Contributors: J. Anthony Koslow, Peter Auster, Odd Aksel Bergstad, J. Murray Roberts, Alex Rogers, Michael Vecchione, Peter Harris, Jake Rice, Patricio Bernal (Co-Lead members) 1. Physical, chemical, and ecological characteristics 1.1 Seamounts Seamounts are predominantly submerged volcanoes, mostly extinct, rising hundreds to thousands of metres above the surrounding seafloor. Some also arise through tectonic uplift. The conventional geological definition includes only features greater than 1000 m in height, with the term “knoll” often used to refer to features 100 – 1000 m in height (Yesson et al., 2011). However, seamounts and knolls do not appear to differ much ecologically, and human activity, such as fishing, focuses on both. We therefore include here all such features with heights > 100 m. Only 6.5 per cent of the deep seafloor has been mapped, so the global number of seamounts must be estimated, usually from a combination of satellite altimetry and multibeam data as well as extrapolation based on size-frequency relationships of seamounts for smaller features. Estimates have varied widely as a result of differences in methodologies as well as changes in the resolution of data. Yesson et al. (2011) identified 33,452 seamount and guyot features > 1000 m in height and 138,412 knolls (100 – 1000 m), whereas Harris et al. (2014) identified 10,234 seamount and guyot features, based on a stricter definition that restricted seamounts to conical forms. Estimates of total abundance range to >100,000 seamounts and to 25 million for features > 100 m in height (Smith 1991; Wessel et al., 2010). -

Microbiology of Seamounts Is Still in Its Infancy
or collective redistirbution of any portion of this article by photocopy machine, reposting, or other means is permitted only with the approval of The approval portionthe ofwith any articlepermitted only photocopy by is of machine, reposting, this means or collective or other redistirbution This article has This been published in MOUNTAINS IN THE SEA Oceanography MICROBIOLOGY journal of The 23, Number 1, a quarterly , Volume OF SEAMOUNTS Common Patterns Observed in Community Structure O ceanography ceanography S BY DAVID EmERSON AND CRAIG L. MOYER ociety. © 2010 by The 2010 by O ceanography ceanography O ceanography ceanography ABSTRACT. Much interest has been generated by the discoveries of biodiversity InTRODUCTION S ociety. ociety. associated with seamounts. The volcanically active portion of these undersea Microbial life is remarkable for its resil- A mountains hosts a remarkably diverse range of unusual microbial habitats, from ience to extremes of temperature, pH, article for use and research. this copy in teaching to granted ll rights reserved. is Permission S ociety. ociety. black smokers rich in sulfur to cooler, diffuse, iron-rich hydrothermal vents. As and pressure, as well its ability to persist S such, seamounts potentially represent hotspots of microbial diversity, yet our and thrive using an amazing number or Th e [email protected] to: correspondence all end understanding of the microbiology of seamounts is still in its infancy. Here, we of organic or inorganic food sources. discuss recent work on the detection of seamount microbial communities and the Nowhere are these traits more evident observation that specific community groups may be indicative of specific geochemical than in the deep ocean. -

Modelling the Distribution of Alfonsino, Beryx Splendens, Over The
Abstract.- Commercial and Modelling the distribution of scientificbottom longline catches of alfonsino, Beryx splendens, from alfonsino, Beryx splendens, over seamounts off New Caledonia were sampled to study length-frequency distributions. A total of 14,674 fish the seamounts of New Caledonia were measured. CPUE of Beryx splendens on two seamounts is mod- Patrick Lehodey elled in terms of length and depth. The data show that mean length in- Paul Marchal creases with depth; this is well de- scribed by a bivariate normal model Rene Grandperrin that estimates catch for a given sea- Centre ORSTOM, BP A5, Noumea, New Caledonia mount. In addition, the data show I that mean length also varies with the depth of the top of seamounts; this is described by a recursive model that is designed to predict ap- proximate catch for any seamount. A bottom longline fishery operated recursive model predicts catch on The limitations of both models are on the seamounts of the Exclusive any seamount. discussed, particularly with regard Economic Zone (EEZ) of New Cale- to temporal variation. donia from February 1988 to July 1991.l Three vessels were involved Material and methods but only one vessel was operated at any given time. The fishing effort, Data which totalled 4,691,635 hooks, fo- Alfonsino were captured with long- cused on five seamounts (B, C, D, line gear (Fig. 2). The main line, J, and K) whose summits are lo- averaging 4,000 m, was held on the cated at depths ranging from 500 1). bottom by means of terminal an- to 750 m (Fig. -
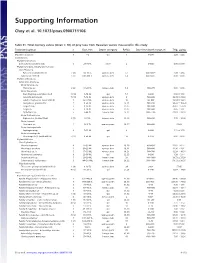
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Beryx Splendens Lowe, 1834
Beryx splendens Lowe, 1834 AphiaID: 126395 SPLENDID ALFONSINO Animalia (Reino) > Chordata (Filo) > Vertebrata (Subfilo) > Gnathostomata (Infrafilo) > Pisces (Superclasse) > Pisces (Superclasse-2) > Actinopterygii (Classe) > Beryciformes (Ordem) > Berycidae (Familia) Heessen, Henk Sinónimos Bryx splendens Lowe, 1834 Referências MARTINS, R.; CARNEIRO, M., 2018. Manual de identificação de peixes ósseos da costa continental portuguesa – Principais Características Diagnosticantes. IPMA, I.P., 204p Fernandes, P., Collette, B., Heessen, H., Smith-Vaniz, W.F. & Herrera, J. 2015. Beryx splendens. The IUCN Red List of Threatened Species 2015: e.T16425354A45791585. Downloaded on 05 August 2019. additional source Froese, R. & D. Pauly (Editors). (2018). FishBase. World Wide Web electronic publication. , available online at http://www.fishbase.org [details] basis of record van der Land, J.; Costello, M.J.; Zavodnik, D.; Santos, R.S.; Porteiro, F.M.; Bailly, N.; 1 Eschmeyer, W.N.; Froese, R. (2001). Pisces, in: Costello, M.J. et al. (Ed.) (2001). European register of marine species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Collection Patrimoines Naturels, 50: pp. 357-374 [details] additional source Gulf of Maine Biogeographic Information System (GMBIS) Electronic Atlas. 2002. November, 2002. [details] additional source Hareide, N.R. and G. Garnes. 1998. The distribution and abundance of deep water fish along the Mid-Atlantic Ridge from 43°N to 61°N. Theme session on deep water fish and fisheries. ICES CM 1998/O:39. [details] additional source Welshman, D., S. Kohler, J. Black and L. Van Guelpen. 2003. An atlas of distributions of Canadian Atlantic fishes. , available online at http://epe.lac-bac.gc.ca/100/205/301/ic/cdc/FishAtlas/default.htm [details] additional source Streftaris, N.; Zenetos, A.; Papathanassiou, E.