Applications of 3D Printing on Craniofacial Bone Repair: a Systematic Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BSC2085L Practice Quiz 2
Preparation for Quiz 2: Bone/cartilage descriptions. Match the name of the bone/cartilage in Table 1 with the description in Table 2: Table 1 Bone/cartilage Table 2 Bone Marking/Description A. Sutures Bones that are longer than they are wide B. Sesamoid Bone Majority of Skeletal Cartilage C. Compact Bone Bones that develop inside Tendons D. Spongy Bone Intervertebral Disks and Knee Joint Cartilage E. Long Bone Smooth and Homogenous Bone Tissue F. Short Bone Found in external Ear and Epiglottis G. Flat Bone Bones that are thin and wafer like H. Irregular Bone Bones that do not fall into another category I. Hyaline Cartilage Bones with lots of open spaces J. Elastic Cartilage These are found between Cranial Bones K. Fibrocartliage Bones that are cube-shaped Preparation for Quiz 2: Bone Markings Match the name of the bone marking in Column 1 with the correct description in Column 2: Column 1 Column 2 A. Condyle Very large, blunt projection (only on femur) Irregularly shaped B. Ramus Arm-like bar of bone C. Crest Round or oval opening D. Epicondyle Narrow ridge of bone E. Tubercle Rounded articular projection F. Tuberosity Sharp Slender Process G. Trochanter Canal-like passageway H. Meatus Shallow Depression I. Fossa Narrow, slit-like opening J. Fissure Raised area on or above a condyle K. Sinus Large rounded projection L. Groove Air filled cavity in a bone M. Head Smooth nearly flat articular surface N. Facet Bony expansion on a narrow neck O. Spine Projection or prominence P. Foramen Narrow ridge smaller than a crest Q. -

Introduction to Anatomy Skeletal System: Bone
INTRODUCTION TO ANATOMY SKELETAL SYSTEM: BONE Foundation block - Anatomy - Lecture 1 Objective Color guide : •At the end of the lecture, students should be able to: Only in boys slides in Green • Define the word “Anatomy” Only in girls slides in Purple important and doctors note in Red • Enumerate the different anatomical fields Extra information in Blue • Describe the anatomical position • Describe different anatomical terms of position & movements as well different anatomical planes • Classify bones according to shape, structure & development • Enumerate different bones of both axial & appendicular skeleton ANATOMY & its Sciences. THE WORD ANATOMY is of GREEK origin meaning cutting up(ana=up,tomy=cutting). Girls slides DEFINITION OF ANATOMY: the science which deals with the study of, The structure & shape of the body parts & their relationships to one another. Boys slides ANATOMICAL SCIENCES: 1. Gross Anatomy: study of the human body with NAKED EYES 2. Microscopic Anatomy(Histology): Study of FINE STRUCTURE (cells & tissues) of the human body with the help of Microscope. 3. Developmental Anatomy (Embryology) 4. Radiologist Anatomy (study of the structure and morphology of the tissues and organs of the body based on their x-ray visualization). 5. Surgical Anatomy (practical) 6. Cross-sectional Anatomy (study of the relationship of the structures of the body by the examination of cross sections of the tissue or organ) 7. Applied Anatomy (study of the structure of the organs of the body as it relates to the diagnosis and treatment of disease) -

Anatomy, Skeletal System Review.Pdf
Name____________________________________Period_____ Anatomy & Physiology Part 1 – Mr. Rizzo, Mr. Romano Skeletal System Review Match/Label the following: (1 point each) 1. Flat Bone 2. Short Bone 3. Irregular Bone 4. Long Bone 5. Axial Skeleton 6. Appendicular Skeleton E – Bones of the upper and lower limbs, shoulder, and hip F – Bones of the skull, vertebral column, and rib cage Match the following: (1 point each) 7. Found in the external ear and the epiglottis A. Hyaline 8. Provides support, flexibility, and resilience, is found in the nose B. Fibrocartilage 9. Contains collagen fibers, found in the menisci of the knee and in intervertebral discs C. Interstitial 10. Growth of cartilage: cells in the perichondrium secrete matrix against the D. Elastic external face of existing cartilage E. Appositional 11. Growth of cartilage: lacunae-bound chondrocytes inside the cartilage divide and secrete new matrix, expanding the cartilage from within. Match the following: (1 point each) 12. Diaphysis 13. Compact Bone 14. Proximal Epiphysis 15. Spongy Bone 16. Distal Epiphysis 17. Medullary Cavity 18. Ephyseal Plate True/False: (1 point each) 19. Bone markings can be the sites of attachment for muscles, ligaments, and tendons. 20. Calcification of cartilage occurs during normal bone growth and old age. 21. A ‘sinus’ is a cavity in a bone. 22. A compact bone has a honeycomb texture of trabeculae filled with yellow bone marrow. 23. Long bones consist of a diaphysis and an epiphysis. 24. Short, irregular, AND flat bones have no diaphysis or epiphyses. 25. Hematopoietic tissue (red marrow) is located in the medullary cavity and all areas of spongy bone in adults. -

Human Anatomy and Physiology
LECTURE NOTES For Nursing Students Human Anatomy and Physiology Nega Assefa Alemaya University Yosief Tsige Jimma University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2003 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2003 by Nega Assefa and Yosief Tsige All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Human Anatomy and Physiology Preface There is a shortage in Ethiopia of teaching / learning material in the area of anatomy and physicalogy for nurses. The Carter Center EPHTI appreciating the problem and promoted the development of this lecture note that could help both the teachers and students. -

Terms to Know
Terms To Know Anatomical Terminology Anterior – front of the animal Caudal – towards the tail of an animal Cranial – towards the head of an animal Deep – further from the surface Distal – part of the limb furthest from the body Dorsal – along the back or uppermost surface Frontal plane – body plane that divides the animal into dorsal and ventral parts Lateral – side of an animal Median – body plane that divides the animal into “equal” right and left halves Posterior – rear of the animal Proximal – part of the limb closest to the body Sagittal – any body plane that is parallel to the median plane Superficial – closer to the surface Transverse – body plane that divides the body into cranial and caudal parts Ventral – along the belly surface Skeletal System Appendicular skeleton – consists of fore and hind limbs Axial skeleton – consists of the skull and vertebrae Comminuted fracture – bone shatters into many pieces Compound fracture – bone breaks through the skin Diaphysis – body of a long bone Endosteum – thin inner layer of bone covering; lines medullary cavity Epiphysis – enlarged ends of long bones Fissure fracture – break along the long axis of a bone Flat bone – plate of bone, i.e. scapula Greenstick fracture – break on one side of a bone, usually due to a bending force Irregular bone – complex and irregularly shaped bone, i.e. vertebrae Long bone – bone longer than it is wide, i.e. humerus, radius, and femur Medullary cavity – space within the bone filled with marrow Metaphysis – joining point of epiphysis and diaphysis Ossification – process by which tissue and cartilage becomes bone Periosteum – thin outer layer of bone covering Sesamoid – small, seed-shaped bone embedded in a tendon, i.e. -

Compact Bone Spongy Bone
Spongy bone Compact bone © 2018 Pearson Education, Inc. 1 (b) Flat bone (sternum) (a) Long bone (humerus) (d) Irregular bone (vertebra), right lateral view (c) Short bone (talus) © 2018 Pearson Education, Inc. 2 Articular cartilage Proximal epiphysis Spongy bone Epiphyseal line Periosteum Compact bone Medullary cavity (lined by endosteum) Diaphysis Distal epiphysis (a) © 2018 Pearson Education, Inc. 3 Trabeculae of spongy bone Osteon (Haversian Perforating system) (Volkmann’s) canal Blood vessel continues into medullary cavity containing marrow Blood vessel Lamellae Compact bone Central (Haversian) canal Perforating (Sharpey’s) fibers Periosteum Periosteal blood vessel (a) © 2018 Pearson Education, Inc. 4 Lamella Osteocyte Canaliculus Lacuna Central Bone matrix (Haversian) canal (b) © 2018 Pearson Education, Inc. 5 Osteon Interstitial lamellae Lacuna Central (Haversian) canal (c) © 2018 Pearson Education, Inc. 6 Articular cartilage Hyaline Spongy cartilage bone New center of bone growth New bone Epiphyseal forming plate cartilage Growth Medullary in bone cavity width Bone starting Invading to replace Growth blood cartilage in bone vessels length New bone Bone collar forming Hyaline Epiphyseal cartilage plate cartilage model In an embryo In a fetus In a child © 2018 Pearson Education, Inc. 7 Bone growth Bone grows in length because: Articular cartilage 1 Cartilage grows here. Epiphyseal plate 2 Cartilage is replaced by bone here. 3 Cartilage grows here. © 2018 Pearson Education, Inc. 8 Bone remodeling Growing shaft is remodeled as: Articular cartilage Epiphyseal plate 1 Bone is resorbed by osteoclasts here. 2 Bone is added (appositional growth) by osteoblasts here. 3 Bone is resorbed by osteoclasts here. © 2018 Pearson Education, Inc. 9 Hematoma External Bony callus callus of spongy bone New Internal blood callus vessels Healed (fibrous fracture tissue and Spongy cartilage) bone trabecula 1 Hematoma 2 Fibrocartilage 3 Bony callus 4 Bone remodeling forms. -

Musculoskeletal Biomechanics, Paul Brinckman, Wolfgang Frobin and Gunnar Leivseth, Thieme, New York 202, 256 Pages, ISBN 1588900800
Dr. Muhammad Wasif Rashid Chaudhary MBBS, MBA,CTQM, CSSGB, CPHQ (Ahalia Hospital) “Biomechanics is the study of the structure and function of biological systems”. Bio= Living Mechanics= Forces and effects This includes studies of the tissues including bone, cartilage, ligament, tendon, muscle, and nerve, at multiple scales ranging from the single cell to whole body. (Bio) Mechanics Deformable Statics Dynamics Fluids Solids Kinematics Kinetics Stress Strain Linear Angular The study of mechanics in the human body divided into 2 areas: Kinematics – study of the variables that describe or quantify motion • Displacement • Velocity • Acceleration Kinetics – study of the variables that cause or influence motion • Forces • Torques • Mass Biomechanists use the principles of mechanics in the analysis of human movement to answer questions such as: 1. How can human performance be enhanced? 2. How can injuries be prevented? 3. How can rehabilitation from injury be expedited? By having an understanding of the principles of analysis in biomechanics and the biomechanical properties of the primary tissues of the musculoskeletal system, we will be able to understand the mechanics of normal movement at each region and to appreciate the effects of impairments on the pathomechanics of movement. Overview Sensory Input- gathering information - To monitor changes occurring inside and outside the body Integration - To process and interpret sensory input and decide if action is needed Motor output - A response to stimuli activates muscles or glands Central Nervous System 1. Brain 2. Spinal cord Peripheral Nervous System – 1- Cranial and 2- Peripheral Two division: 1. Sensory division (afferent) – carry information to the CN system 2. Motor division (efferent) – carry impulses away from the CNS • Somatic (voluntary) system • Autonomic (involuntary) system Cells are grouped into two functional categories - Neurons Do all of the major functions on their own, are 1. -

Biodegradable Materials for Bone Defect Repair Shuai Wei1, Jian-Xiong Ma1, Lai Xu2, Xiao-Song Gu2* and Xin-Long Ma1*
Wei et al. Military Medical Research (2020) 7:54 https://doi.org/10.1186/s40779-020-00280-6 REVIEW Open Access Biodegradable materials for bone defect repair Shuai Wei1, Jian-Xiong Ma1, Lai Xu2, Xiao-Song Gu2* and Xin-Long Ma1* Abstract Compared with non-degradable materials, biodegradable biomaterials play an increasingly important role in the repairing of severe bone defects, and have attracted extensive attention from researchers. In the treatment of bone defects, scaffolds made of biodegradable materials can provide a crawling bridge for new bone tissue in the gap and a platform for cells and growth factors to play a physiological role, which will eventually be degraded and absorbed in the body and be replaced by the new bone tissue. Traditional biodegradable materials include polymers, ceramics and metals, which have been used in bone defect repairing for many years. Although these materials have more or fewer shortcomings, they are still the cornerstone of our development of a new generation of degradable materials. With the rapid development of modern science and technology, in the twenty-first century, more and more kinds of new biodegradable materials emerge in endlessly, such as new intelligent micro- nano materials and cell-based products. At the same time, there are many new fabrication technologies of improving biodegradable materials, such as modular fabrication, 3D and 4D printing, interface reinforcement and nanotechnology. This review will introduce various kinds of biodegradable materials commonly used in bone defect repairing, especially the newly emerging materials and their fabrication technology in recent years, and look forward to the future research direction, hoping to provide researchers in the field with some inspiration and reference. -

FIPAT-TA2-Part-2.Pdf
TERMINOLOGIA ANATOMICA Second Edition (2.06) International Anatomical Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TA2, PART II Contents: Systemata musculoskeletalia Musculoskeletal systems Caput II: Ossa Chapter 2: Bones Caput III: Juncturae Chapter 3: Joints Caput IV: Systema musculare Chapter 4: Muscular system Bibliographic Reference Citation: FIPAT. Terminologia Anatomica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, 2019 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Anatomica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput II: OSSA Chapter 2: BONES Latin term Latin synonym UK English US English English synonym Other 351 Systemata Musculoskeletal Musculoskeletal musculoskeletalia systems systems -

The Musculoskeletal System
DOCUMENT RESUME '.' .. 2 . ED 213 965- 2 . CE 011 771 1' 'TITLE. The Mukuldskeletal System [and] Instructor's Guide: The Musculoskeletal System. Health Occupations. ^ Education Module: Instructional Materials in Atttomy ( . and Physiology for Pennsylvania Health Occupations . Programs. INSTITUTION 'National Evaluation Systems, Inc., Amherst, Mass. SPONS AGENCY-, Pennsylvania State Dept. of Education, Harrisburg. Bureau of Vocational and Technical Education,. PUB DATE Jun 79 . c NOTE ". 64p.; for related documents see listing in note of CE 031 758. 0 EDRS PRICE MF01/PC03 Plus Postage. 'DESCRIPTORS *Allied Health OccupatiOns Education; *Anatomy; ". Behavioral Objectives; *Individualized Instruction; *Learning Activities; Learning Modules; Medical -Vocabulary; '*Physiology; Postsecondary Education; , Pretests Posttests; Programed -Instructional, Materials; Secondary Education; Self Evaluation - (Individuals).; Teaching Methods IDENTIFIERS): *Muscular System; Pennsylvania; *Skeletal Systems 4. , 4 ABSTRACT - 4 04 This Module on the musculoskeletal system is one of modules designed tor individualized instruction-in-health occUpations education progrqts at' both the secondary and postsecondary levels. It is.,part of an eight-unii miniseries on anatOmy and phySiology within the Apaies of 17 Moddles. Following a preface which explains to the student how to use the module; the unit consistsof a pretest with afiswer, seven'sections (information sheets)' with their goals (e.g., classify different -types of bone), optional activities (e.g., on diagrams of the skeleton, draw the: major muscles of the body), and posttests, and a glossary of. terms. Tojics covered .in the unitare introddctiol to the ikeletal systemf axial skeleton, appendicutaskeleton, introduction tothe muscular system, major muscles of the body, Upporti!'tg structures of the mRsculoskeletal system, and movements. An accompanying instructor's Ale contains suggestions for.using,the modttle and. -
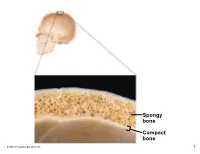
Compact Bone Spongy Bone
Spongy bone Compact bone © 2018 Pearson Education, Inc. 1 (b) Flat bone (sternum) (a) Long bone (humerus) (d) Irregular bone (vertebra), right lateral view (c) Short bone (talus) © 2018 Pearson Education, Inc. 2 Articular cartilage Proximal epiphysis Spongy bone Epiphyseal line Periosteum Compact bone Medullary cavity (lined by endosteum) Diaphysis Distal epiphysis (a) © 2018 Pearson Education, Inc. 3 Trabeculae of spongy bone Osteon (Haversian Perforating system) (Volkmann’s) canal Blood vessel continues into medullary cavity containing marrow Blood vessel Lamellae Compact bone Central (Haversian) canal Perforating (Sharpey’s) fibers Periosteum Periosteal blood vessel (a) © 2018 Pearson Education, Inc. 4 Lamella Osteocyte Canaliculus Lacuna Central Bone matrix (Haversian) canal (b) © 2018 Pearson Education, Inc. 5 Osteon Interstitial lamellae Lacuna Central (Haversian) canal (c) © 2018 Pearson Education, Inc. 6 Articular cartilage Hyaline Spongy cartilage bone New center of bone growth New bone Epiphyseal forming plate cartilage Growth Medullary in bone cavity width Bone starting Invading to replace Growth blood cartilage in bone vessels length New bone Bone collar forming Hyaline Epiphyseal cartilage plate cartilage model In an embryo In a fetus In a child © 2018 Pearson Education, Inc. 7 Bone growth Bone grows in length because: Articular cartilage 1 Cartilage grows here. Epiphyseal plate 2 Cartilage is replaced by bone here. 3 Cartilage grows here. © 2018 Pearson Education, Inc. 8 Bone remodeling Growing shaft is remodeled as: Articular cartilage Epiphyseal plate 1 Bone is resorbed by osteoclasts here. 2 Bone is added (appositional growth) by osteoblasts here. 3 Bone is resorbed by osteoclasts here. © 2018 Pearson Education, Inc. 9 Hematoma External Bony callus callus of spongy bone New Internal blood callus vessels Healed (fibrous fracture tissue and Spongy cartilage) bone trabecula 1 Hematoma 2 Fibrocartilage 3 Bony callus 4 Bone remodeling forms. -

The Skeletal System
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC © BraunS/E+/Getty Images NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION CHAPTER 2 © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION The Skeletal System © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FORCHAPTER SALE OR OBJECTIVESDISTRIBUTION NOT FOR SALE OR DISTRIBUTION After completing this chapter, the student will be able to: 1. describe general anatomy of a bone; 2. recall the types of© bone Jones classified & Bartlett by shape; Learning, LLC © Jones & Bartlett Learning, LLC 3. employ terminologyNOT related FOR to anatomicalSALE OR features DISTRIBUTION of bones; NOT FOR SALE OR DISTRIBUTION 4. realize the functional and structural classifications of joints; and 5. recognize the types of synovial joints. © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOThe skeletal FOR systemSALE ORis comprised DISTRIBUTION of several The bonesNOT are arrangedFOR SALE as a relatively OR DISTRIBUTION solid frame- organs called bones (FIGURE 2.1). The main func- work which supports all the other organs directly or tions of the skeletal system and its bones are: indirectly. It is like the steel beams in a commercial T building. The walls directly attach to the beams. This 1. support (framework), © Jones & Bartlett2. prot ection,Learning, and LLC © Jonesmakes a &solid Bartlett surface Learning,for pictures to LLC hang from and be supported by the walls. The bones are arranged in a NOT FOR SALE3.