Statistical Methods for Overdispersion in Mrna-Seq Count Data Hui Zhang*, Stanley B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Introduction to Poisson Regression Russ Lavery, K&L Consulting Services, King of Prussia, PA, U.S.A
NESUG 2010 Statistics and Analysis An Animated Guide: An Introduction To Poisson Regression Russ Lavery, K&L Consulting Services, King of Prussia, PA, U.S.A. ABSTRACT: This paper will be a brief introduction to Poisson regression (theory, steps to be followed, complications and interpretation) via a worked example. It is hoped that this will increase motivation towards learning this useful statistical technique. INTRODUCTION: Poisson regression is available in SAS through the GENMOD procedure (generalized modeling). It is appropriate when: 1) the process that generates the conditional Y distributions would, theoretically, be expected to be a Poisson random process and 2) when there is no evidence of overdispersion and 3) when the mean of the marginal distribution is less than ten (preferably less than five and ideally close to one). THE POISSON DISTRIBUTION: The Poison distribution is a discrete Percent of observations where the random variable X is expected distribution and is appropriate for to have the value x, given that the Poisson distribution has a mean modeling counts of observations. of λ= P(X=x, λ ) = (e - λ * λ X) / X! Counts are observed cases, like the 0.4 count of measles cases in cities. You λ can simply model counts if all data 0.35 were collected in the same measuring 0.3 unit (e.g. the same number of days or 0.3 0.5 0.8 same number of square feet). 0.25 λ 1 0.2 3 You can use the Poisson Distribution = 5 for modeling rates (rates are counts 0.15 20 per unit) if the units of collection were 8 different. -

Parametric Models
An Introduction to Event History Analysis Oxford Spring School June 18-20, 2007 Day Two: Regression Models for Survival Data Parametric Models We’ll spend the morning introducing regression-like models for survival data, starting with fully parametric (distribution-based) models. These tend to be very widely used in social sciences, although they receive almost no use outside of that (e.g., in biostatistics). A General Framework (That Is, Some Math) Parametric models are continuous-time models, in that they assume a continuous parametric distribution for the probability of failure over time. A general parametric duration model takes as its starting point the hazard: f(t) h(t) = (1) S(t) As we discussed yesterday, the density is the probability of the event (at T ) occurring within some differentiable time-span: Pr(t ≤ T < t + ∆t) f(t) = lim . (2) ∆t→0 ∆t The survival function is equal to one minus the CDF of the density: S(t) = Pr(T ≥ t) Z t = 1 − f(t) dt 0 = 1 − F (t). (3) This means that another way of thinking of the hazard in continuous time is as a conditional limit: Pr(t ≤ T < t + ∆t|T ≥ t) h(t) = lim (4) ∆t→0 ∆t i.e., the conditional probability of the event as ∆t gets arbitrarily small. 1 A General Parametric Likelihood For a set of observations indexed by i, we can distinguish between those which are censored and those which aren’t... • Uncensored observations (Ci = 1) tell us both about the hazard of the event, and the survival of individuals prior to that event. -

Overdispersion, and How to Deal with It in R and JAGS (Requires R-Packages AER, Coda, Lme4, R2jags, Dharma/Devtools) Carsten F
Overdispersion, and how to deal with it in R and JAGS (requires R-packages AER, coda, lme4, R2jags, DHARMa/devtools) Carsten F. Dormann 07 December, 2016 Contents 1 Introduction: what is overdispersion? 1 2 Recognising (and testing for) overdispersion 1 3 “Fixing” overdispersion 5 3.1 Quasi-families . .5 3.2 Different distribution (here: negative binomial) . .6 3.3 Observation-level random effects (OLRE) . .8 4 Overdispersion in JAGS 12 1 Introduction: what is overdispersion? Overdispersion describes the observation that variation is higher than would be expected. Some distributions do not have a parameter to fit variability of the observation. For example, the normal distribution does that through the parameter σ (i.e. the standard deviation of the model), which is constant in a typical regression. In contrast, the Poisson distribution has no such parameter, and in fact the variance increases with the mean (i.e. the variance and the mean have the same value). In this latter case, for an expected value of E(y) = 5, we also expect that the variance of observed data points is 5. But what if it is not? What if the observed variance is much higher, i.e. if the data are overdispersed? (Note that it could also be lower, underdispersed. This is less often the case, and not all approaches below allow for modelling underdispersion, but some do.) Overdispersion arises in different ways, most commonly through “clumping”. Imagine the number of seedlings in a forest plot. Depending on the distance to the source tree, there may be many (hundreds) or none. The same goes for shooting stars: either the sky is empty, or littered with shooting stars. -

Poisson Versus Negative Binomial Regression
Handling Count Data The Negative Binomial Distribution Other Applications and Analysis in R References Poisson versus Negative Binomial Regression Randall Reese Utah State University [email protected] February 29, 2016 Randall Reese Poisson and Neg. Binom Handling Count Data The Negative Binomial Distribution Other Applications and Analysis in R References Overview 1 Handling Count Data ADEM Overdispersion 2 The Negative Binomial Distribution Foundations of Negative Binomial Distribution Basic Properties of the Negative Binomial Distribution Fitting the Negative Binomial Model 3 Other Applications and Analysis in R 4 References Randall Reese Poisson and Neg. Binom Handling Count Data The Negative Binomial Distribution ADEM Other Applications and Analysis in R Overdispersion References Count Data Randall Reese Poisson and Neg. Binom Handling Count Data The Negative Binomial Distribution ADEM Other Applications and Analysis in R Overdispersion References Count Data Data whose values come from Z≥0, the non-negative integers. Classic example is deaths in the Prussian army per year by horse kick (Bortkiewicz) Example 2 of Notes 5. (Number of successful \attempts"). Randall Reese Poisson and Neg. Binom Handling Count Data The Negative Binomial Distribution ADEM Other Applications and Analysis in R Overdispersion References Poisson Distribution Support is the non-negative integers. (Count data). Described by a single parameter λ > 0. When Y ∼ Poisson(λ), then E(Y ) = Var(Y ) = λ Randall Reese Poisson and Neg. Binom Handling Count Data The Negative Binomial Distribution ADEM Other Applications and Analysis in R Overdispersion References Acute Disseminated Encephalomyelitis Acute Disseminated Encephalomyelitis (ADEM) is a neurological, immune disorder in which widespread inflammation of the brain and spinal cord damages tissue known as white matter. -
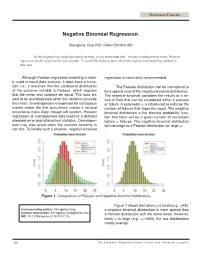
Negative Binomial Regression
STATISTICS COLUMN Negative Binomial Regression Shengping Yang PhD ,Gilbert Berdine MD In the hospital stay study discussed recently, it was mentioned that “in case overdispersion exists, Poisson regression model might not be appropriate.” I would like to know more about the appropriate modeling method in that case. Although Poisson regression modeling is wide- regression is commonly recommended. ly used in count data analysis, it does have a limita- tion, i.e., it assumes that the conditional distribution The Poisson distribution can be considered to of the outcome variable is Poisson, which requires be a special case of the negative binomial distribution. that the mean and variance be equal. The data are The negative binomial considers the results of a se- said to be overdispersed when the variance exceeds ries of trials that can be considered either a success the mean. Overdispersion is expected for contagious or failure. A parameter ψ is introduced to indicate the events where the first occurrence makes a second number of failures that stops the count. The negative occurrence more likely, though still random. Poisson binomial distribution is the discrete probability func- regression of overdispersed data leads to a deflated tion that there will be a given number of successes standard error and inflated test statistics. Overdisper- before ψ failures. The negative binomial distribution sion may also result when the success outcome is will converge to a Poisson distribution for large ψ. not rare. To handle such a situation, negative binomial 0 5 10 15 20 25 0 5 10 15 20 25 Figure 1. Comparison of Poisson and negative binomial distributions. -

Generalized Linear Models
CHAPTER 6 Generalized linear models 6.1 Introduction Generalized linear modeling is a framework for statistical analysis that includes linear and logistic regression as special cases. Linear regression directly predicts continuous data y from a linear predictor Xβ = β0 + X1β1 + + Xkβk.Logistic regression predicts Pr(y =1)forbinarydatafromalinearpredictorwithaninverse-··· logit transformation. A generalized linear model involves: 1. A data vector y =(y1,...,yn) 2. Predictors X and coefficients β,formingalinearpredictorXβ 1 3. A link function g,yieldingavectoroftransformeddataˆy = g− (Xβ)thatare used to model the data 4. A data distribution, p(y yˆ) | 5. Possibly other parameters, such as variances, overdispersions, and cutpoints, involved in the predictors, link function, and data distribution. The options in a generalized linear model are the transformation g and the data distribution p. In linear regression,thetransformationistheidentity(thatis,g(u) u)and • the data distribution is normal, with standard deviation σ estimated from≡ data. 1 1 In logistic regression,thetransformationistheinverse-logit,g− (u)=logit− (u) • (see Figure 5.2a on page 80) and the data distribution is defined by the proba- bility for binary data: Pr(y =1)=y ˆ. This chapter discusses several other classes of generalized linear model, which we list here for convenience: The Poisson model (Section 6.2) is used for count data; that is, where each • data point yi can equal 0, 1, 2, ....Theusualtransformationg used here is the logarithmic, so that g(u)=exp(u)transformsacontinuouslinearpredictorXiβ to a positivey ˆi.ThedatadistributionisPoisson. It is usually a good idea to add a parameter to this model to capture overdis- persion,thatis,variationinthedatabeyondwhatwouldbepredictedfromthe Poisson distribution alone. -

Robust Estimation on a Parametric Model Via Testing 3 Estimators
Bernoulli 22(3), 2016, 1617–1670 DOI: 10.3150/15-BEJ706 Robust estimation on a parametric model via testing MATHIEU SART 1Universit´ede Lyon, Universit´eJean Monnet, CNRS UMR 5208 and Institut Camille Jordan, Maison de l’Universit´e, 10 rue Tr´efilerie, CS 82301, 42023 Saint-Etienne Cedex 2, France. E-mail: [email protected] We are interested in the problem of robust parametric estimation of a density from n i.i.d. observations. By using a practice-oriented procedure based on robust tests, we build an estimator for which we establish non-asymptotic risk bounds with respect to the Hellinger distance under mild assumptions on the parametric model. We show that the estimator is robust even for models for which the maximum likelihood method is bound to fail. A numerical simulation illustrates its robustness properties. When the model is true and regular enough, we prove that the estimator is very close to the maximum likelihood one, at least when the number of observations n is large. In particular, it inherits its efficiency. Simulations show that these two estimators are almost equal with large probability, even for small values of n when the model is regular enough and contains the true density. Keywords: parametric estimation; robust estimation; robust tests; T-estimator 1. Introduction We consider n independent and identically distributed random variables X1,...,Xn de- fined on an abstract probability space (Ω, , P) with values in the measure space (X, ,µ). E F We suppose that the distribution of Xi admits a density s with respect to µ and aim at estimating s by using a parametric approach. -

Modeling Overdispersion with the Normalized Tempered Stable Distribution
Modeling overdispersion with the normalized tempered stable distribution M. Kolossiatisa, J.E. Griffinb, M. F. J. Steel∗,c aDepartment of Hotel and Tourism Management, Cyprus University of Technology, P.O. Box 50329, 3603 Limassol, Cyprus. bInstitute of Mathematics, Statistics and Actuarial Science, University of Kent, Canterbury, CT2 7NF, U.K. cDepartment of Statistics, University of Warwick, Coventry, CV4 7AL, U.K. Abstract A multivariate distribution which generalizes the Dirichlet distribution is intro- duced and its use for modeling overdispersion in count data is discussed. The distribution is constructed by normalizing a vector of independent tempered sta- ble random variables. General formulae for all moments and cross-moments of the distribution are derived and they are found to have similar forms to those for the Dirichlet distribution. The univariate version of the distribution can be used as a mixing distribution for the success probability of a binomial distribution to define an alternative to the well-studied beta-binomial distribution. Examples of fitting this model to simulated and real data are presented. Keywords: Distribution on the unit simplex; Mice fetal mortality; Mixed bino- mial; Normalized random measure; Overdispersion 1. Introduction In many experiments we observe data as the number of observations in a sam- ple with some property. The binomial distribution is a natural model for this type of data. However, the data are often found to be overdispersed relative to that ∗Corresponding author. Tel.: +44-2476-523369; fax: +44-2476-524532 Email addresses: [email protected] (M. Kolossiatis), [email protected] (J.E. Griffin), [email protected] (M. -

A PARTIALLY PARAMETRIC MODEL 1. Introduction a Notable
A PARTIALLY PARAMETRIC MODEL DANIEL J. HENDERSON AND CHRISTOPHER F. PARMETER Abstract. In this paper we propose a model which includes both a known (potentially) nonlinear parametric component and an unknown nonparametric component. This approach is feasible given that we estimate the finite sample parameter vector and the bandwidths simultaneously. We show that our objective function is asymptotically equivalent to the individual objective criteria for the parametric parameter vector and the nonparametric function. In the special case where the parametric component is linear in parameters, our single-step method is asymptotically equivalent to the two-step partially linear model esti- mator in Robinson (1988). Monte Carlo simulations support the asymptotic developments and show impressive finite sample performance. We apply our method to the case of a partially constant elasticity of substitution production function for an unbalanced sample of 134 countries from 1955-2011 and find that the parametric parameters are relatively stable for different nonparametric control variables in the full sample. However, we find substan- tial parameter heterogeneity between developed and developing countries which results in important differences when estimating the elasticity of substitution. 1. Introduction A notable development in applied economic research over the last twenty years is the use of the partially linear regression model (Robinson 1988) to study a variety of phenomena. The enthusiasm for the partially linear model (PLM) is not confined to -

Variance Partitioning in Multilevel Logistic Models That Exhibit Overdispersion
J. R. Statist. Soc. A (2005) 168, Part 3, pp. 599–613 Variance partitioning in multilevel logistic models that exhibit overdispersion W. J. Browne, University of Nottingham, UK S. V. Subramanian, Harvard School of Public Health, Boston, USA K. Jones University of Bristol, UK and H. Goldstein Institute of Education, London, UK [Received July 2002. Final revision September 2004] Summary. A common application of multilevel models is to apportion the variance in the response according to the different levels of the data.Whereas partitioning variances is straight- forward in models with a continuous response variable with a normal error distribution at each level, the extension of this partitioning to models with binary responses or to proportions or counts is less obvious. We describe methodology due to Goldstein and co-workers for appor- tioning variance that is attributable to higher levels in multilevel binomial logistic models. This partitioning they referred to as the variance partition coefficient. We consider extending the vari- ance partition coefficient concept to data sets when the response is a proportion and where the binomial assumption may not be appropriate owing to overdispersion in the response variable. Using the literacy data from the 1991 Indian census we estimate simple and complex variance partition coefficients at multiple levels of geography in models with significant overdispersion and thereby establish the relative importance of different geographic levels that influence edu- cational disparities in India. Keywords: Contextual variation; Illiteracy; India; Multilevel modelling; Multiple spatial levels; Overdispersion; Variance partition coefficient 1. Introduction Multilevel regression models (Goldstein, 2003; Bryk and Raudenbush, 1992) are increasingly being applied in many areas of quantitative research. -

Options for Development of Parametric Probability Distributions for Exposure Factors
EPA/600/R-00/058 July 2000 www.epa.gov/ncea Options for Development of Parametric Probability Distributions for Exposure Factors National Center for Environmental Assessment-Washington Office Office of Research and Development U.S. Environmental Protection Agency Washington, DC 1 Introduction The EPA Exposure Factors Handbook (EFH) was published in August 1997 by the National Center for Environmental Assessment of the Office of Research and Development (EPA/600/P-95/Fa, Fb, and Fc) (U.S. EPA, 1997a). Users of the Handbook have commented on the need to fit distributions to the data in the Handbook to assist them when applying probabilistic methods to exposure assessments. This document summarizes a system of procedures to fit distributions to selected data from the EFH. It is nearly impossible to provide a single distribution that would serve all purposes. It is the responsibility of the assessor to determine if the data used to derive the distributions presented in this report are representative of the population to be assessed. The system is based on EPA’s Guiding Principles for Monte Carlo Analysis (U.S. EPA, 1997b). Three factors—drinking water, population mobility, and inhalation rates—are used as test cases. A plan for fitting distributions to other factors is currently under development. EFH data summaries are taken from many different journal publications, technical reports, and databases. Only EFH tabulated data summaries were analyzed, and no attempt was made to obtain raw data from investigators. Since a variety of summaries are found in the EFH, it is somewhat of a challenge to define a comprehensive data analysis strategy that will cover all cases. -

Exponential Families and Theoretical Inference
EXPONENTIAL FAMILIES AND THEORETICAL INFERENCE Bent Jørgensen Rodrigo Labouriau August, 2012 ii Contents Preface vii Preface to the Portuguese edition ix 1 Exponential families 1 1.1 Definitions . 1 1.2 Analytical properties of the Laplace transform . 11 1.3 Estimation in regular exponential families . 14 1.4 Marginal and conditional distributions . 17 1.5 Parametrizations . 20 1.6 The multivariate normal distribution . 22 1.7 Asymptotic theory . 23 1.7.1 Estimation . 25 1.7.2 Hypothesis testing . 30 1.8 Problems . 36 2 Sufficiency and ancillarity 47 2.1 Sufficiency . 47 2.1.1 Three lemmas . 48 2.1.2 Definitions . 49 2.1.3 The case of equivalent measures . 50 2.1.4 The general case . 53 2.1.5 Completeness . 56 2.1.6 A result on separable σ-algebras . 59 2.1.7 Sufficiency of the likelihood function . 60 2.1.8 Sufficiency and exponential families . 62 2.2 Ancillarity . 63 2.2.1 Definitions . 63 2.2.2 Basu's Theorem . 65 2.3 First-order ancillarity . 67 2.3.1 Examples . 67 2.3.2 Main results . 69 iii iv CONTENTS 2.4 Problems . 71 3 Inferential separation 77 3.1 Introduction . 77 3.1.1 S-ancillarity . 81 3.1.2 The nonformation principle . 83 3.1.3 Discussion . 86 3.2 S-nonformation . 91 3.2.1 Definition . 91 3.2.2 S-nonformation in exponential families . 96 3.3 G-nonformation . 99 3.3.1 Transformation models . 99 3.3.2 Definition of G-nonformation . 103 3.3.3 Cox's proportional risks model .