Dispersal at Hydrothermal Vents: a Summary of Recent Progress
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
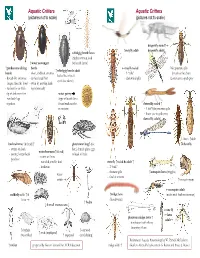
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Visceral and Cutaneous Larva Migrans PAUL C
Visceral and Cutaneous Larva Migrans PAUL C. BEAVER, Ph.D. AMONG ANIMALS in general there is a In the development of our concepts of larva II. wide variety of parasitic infections in migrans there have been four major steps. The which larval stages migrate through and some¬ first, of course, was the discovery by Kirby- times later reside in the tissues of the host with¬ Smith and his associates some 30 years ago of out developing into fully mature adults. When nematode larvae in the skin of patients with such parasites are found in human hosts, the creeping eruption in Jacksonville, Fla. (6). infection may be referred to as larva migrans This was followed immediately by experi¬ although definition of this term is becoming mental proof by numerous workers that the increasingly difficult. The organisms impli¬ larvae of A. braziliense readily penetrate the cated in infections of this type include certain human skin and produce severe, typical creep¬ species of arthropods, flatworms, and nema¬ ing eruption. todes, but more especially the nematodes. From a practical point of view these demon¬ As generally used, the term larva migrans strations were perhaps too conclusive in that refers particularly to the migration of dog and they encouraged the impression that A. brazil¬ cat hookworm larvae in the human skin (cu¬ iense was the only cause of creeping eruption, taneous larva migrans or creeping eruption) and detracted from equally conclusive demon¬ and the migration of dog and cat ascarids in strations that other species of nematode larvae the viscera (visceral larva migrans). In a still have the ability to produce similarly the pro¬ more restricted sense, the terms cutaneous larva gressive linear lesions characteristic of creep¬ migrans and visceral larva migrans are some¬ ing eruption. -

Description of Map Units Northeast Asia Geodynamics Map
DESCRIPTION OF MAP UNITS NORTHEAST ASIA GEODYNAMICS MAP OVERLAP ASSEMBLAGES (Arranged alphabetically by map symbol) ad Adycha intermountain sedimentary basin (Miocene and Pliocene) (Yakutia) Basin forms a discontinuous chain along the foot of southwestern slope of Chersky Range in the Yana and Adycha Rivers basins. Contain Miocene and Pliocene sandstone, pebble gravel conglomerate, claystone, and minor boulder gravel conglomerate that range up to 400 m thick. REFERENCES: Grinenko and others, 1998. ag Agul (Rybinsk) molasse basin (Middle Devonian to Early Carboniferous) (Eastern Sayan) Consists of Middle Devonian through Early Carboniferous aerial and lacustrine sand-silt-mudstone, conglomerate, marl, and limestone with fauna and flora. Tuff, tuffite, and tuffaceous rock occur in Early Carboniferous sedimentary rocks. Ranges up to 2,000 m thick in southwestern margin of basin. Unconformably overlaps Early Devonian rocks of South Siberian volcanic-plutonic belt and Precambrian and early Paleozoic rocks of the Siberian Platform and surrounding fold belts. REFERENCES: Yanov, 1956; Graizer, Borovskaya, 1964. ags Argun sedimentary basin (Early Paleozoic) (Northeastern China) Occurs east of the Argun River in a discontinuously exposed, northeast-trending belt and consists of Cambrian and Ordovician marine, terrigenous detrital, and carbonate rocks. Cambrian units are composed of of feldspar- quartz sandstone, siltstone, shale and limestone and contain abundant Afaciacyathus sp., Bensocyathus sp., Robustocyathus yavorskii, Archaeocyathus yavorskii(Vologalin), Ethomophyllum hinganense Gu,o and other fossils. Ordovicain units consist of feldspar-quartz sandstone, siltstone, fine-grained sandstone and phylitic siltstone, and interlayered metamorphosed muddy siltstone and fine-grained sandstone with brachiopods, corals, and trilobites. Total thickness ranges up to 4,370 m. Basin unconformably overlies the Argunsky metamorphic terrane. -

Evolution of Direct-Developing Larvae: Selection Vs Loss Margaret Snoke Smith,1 Kirk S
Hypotheses Evolution of direct-developing larvae: selection vs loss Margaret Snoke Smith,1 Kirk S. Zigler,2 and Rudolf A. Raff1,3* Summary echinoderms, sea urchins, reveal that a majority of sea urchin Observations of a sea urchin larvae show that most species exhibit one of two life history strategies (Fig. 1).(10) species adopt one of two life history strategies. One strategy is to make numerous small eggs, which develop One strategy is to make many small eggs that develop into a into a larva with a required feeding period in the water larva that must feed and grow for weeks or months in the water column before metamorphosis. In contrast, the second column before metamorphosis. In the water column, these strategy is to make fewer large eggs with a larva that does larvae are dependent on plankton abundance for food and are not feed, which reduces the time to metamorphosis and subject to high levels of predation.(11) The other strategy thus the time spent in the water column. The larvae associated with each strategy have distinct morpholo- involves increasing egg size and maternal provisioning, which gies and developmental processes that reflect their decreases the reliance on planktonic food sources and the feeding requirements, so that those that feed exhibit time to metamorphosis, thus minimizing larval mortality. indirect development with a complex larva, and those that However, assuming finite resources for egg production, do not feed form a morphologically simplified larva and increasing egg size also reduces the total number of eggs exhibit direct development. Phylogenetic studies show that, in sea urchins, a feeding larva, the pluteus, is the produced. -

Did the 1999 Earthquake Swarm on Gakkel Ridge Open a Volcanic Conduit? a Detailed Teleseismic Data Analysis Carsten Riedel, Vera Schlindwein
Did the 1999 earthquake swarm on Gakkel Ridge open a volcanic conduit? A detailed teleseismic data analysis Carsten Riedel, Vera Schlindwein To cite this version: Carsten Riedel, Vera Schlindwein. Did the 1999 earthquake swarm on Gakkel Ridge open a volcanic conduit? A detailed teleseismic data analysis. Journal of Seismology, Springer Verlag, 2009, 14 (3), pp.505-522. 10.1007/s10950-009-9179-6. hal-00537995 HAL Id: hal-00537995 https://hal.archives-ouvertes.fr/hal-00537995 Submitted on 20 Nov 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. J Seismol (2010) 14:505–522 DOI 10.1007/s10950-009-9179-6 ORIGINAL ARTICLE Did the 1999 earthquake swarm on Gakkel Ridge open a volcanic conduit? A detailed teleseismic data analysis Carsten Riedel · Vera Schlindwein Received: 19 February 2009 / Accepted: 2 November 2009 / Published online: 20 November 2009 © Springer Science + Business Media B.V. 2009 Abstract In 1999, a seismic swarm of 237 tele- ascending toward the potential conduit during the seismically recorded events marked a submarine beginning of April 1999, indicating an opening of eruption along the Arctic Gakkel Ridge, later on the vent. -

Hookworm-Related Cutaneous Larva Migrans
326 Hookworm-Related Cutaneous Larva Migrans Patrick Hochedez , MD , and Eric Caumes , MD Département des Maladies Infectieuses et Tropicales, Hôpital Pitié-Salpêtrière, Paris, France DOI: 10.1111/j.1708-8305.2007.00148.x Downloaded from https://academic.oup.com/jtm/article/14/5/326/1808671 by guest on 27 September 2021 utaneous larva migrans (CLM) is the most fre- Risk factors for developing HrCLM have specifi - Cquent travel-associated skin disease of tropical cally been investigated in one outbreak in Canadian origin. 1,2 This dermatosis fi rst described as CLM by tourists: less frequent use of protective footwear Lee in 1874 was later attributed to the subcutane- while walking on the beach was signifi cantly associ- ous migration of Ancylostoma larvae by White and ated with a higher risk of developing the disease, Dove in 1929. 3,4 Since then, this skin disease has also with a risk ratio of 4. Moreover, affected patients been called creeping eruption, creeping verminous were somewhat younger than unaffected travelers dermatitis, sand worm eruption, or plumber ’ s itch, (36.9 vs 41.2 yr, p = 0.014). There was no correla- which adds to the confusion. It has been suggested tion between the reported amount of time spent on to name this disease hookworm-related cutaneous the beach and the risk of developing CLM. Consid- larva migrans (HrCLM).5 ering animals in the neighborhood, 90% of the Although frequent, this tropical dermatosis is travelers in that study reported seeing cats on the not suffi ciently well known by Western physicians, beach and around the hotel area, and only 1.5% and this can delay diagnosis and effective treatment. -

Evolutionary Developmental Biology 573
EVOC20 29/08/2003 11:15 AM Page 572 Evolutionary 20 Developmental Biology volutionary developmental biology, now often known Eas “evo-devo,” is the study of the relation between evolution and development. The relation between evolution and development has been the subject of research for many years, and the chapter begins by looking at some classic ideas. However, the subject has been transformed in recent years as the genes that control development have begun to be identified. This chapter looks at how changes in these developmental genes, such as changes in their spatial or temporal expression in the embryo, are associated with changes in adult morphology. The origin of a set of genes controlling development may have opened up new and more flexible ways in which evolution could occur: life may have become more “evolvable.” EVOC20 29/08/2003 11:15 AM Page 573 CHAPTER 20 / Evolutionary Developmental Biology 573 20.1 Changes in development, and the genes controlling development, underlie morphological evolution Morphological structures, such as heads, legs, and tails, are produced in each individual organism by development. The organism begins life as a single cell. The organism grows by cell division, and the various cell types (bone cells, skin cells, and so on) are produced by differentiation within dividing cell lines. When one species evolves into Morphological evolution is driven another, with a changed morphological form, the developmental process must have by developmental evolution changed too. If the descendant species has longer legs, it is because the developmental process that produces legs has been accelerated, or extended over time. -

Effusive and Explosive Volcanism on the Ultraslow-Spreading Gakkel Ridge, 85�E
Article Volume 13, Number 10 6 October 2012 Q10005, doi:10.1029/2012GC004187 ISSN: 1525-2027 Effusive and explosive volcanism on the ultraslow-spreading Gakkel Ridge, 85E Claire W. Pontbriand MIT-WHOI Joint Program in Oceanography, 266 Woods Hole Road, MS 24, Woods Hole, Massachusetts 02543, USA ([email protected]) S. Adam Soule, Robert A. Sohn, Susan E. Humphris, Clayton Kunz, and Hanumant Singh Woods Hole Oceanographic Institution, 266 Woods Hole Road, MS 24, Woods Hole, Massachusetts 02543, USA ([email protected]; [email protected]; [email protected]; [email protected]; [email protected]) Ko-ichi Nakamura Institute of Geology and Geoinformation, National Institute of Advanced Industrial Science and Technology, 1-1-1 Higashi, Tsukuba 305-8567, Japan ([email protected]) Martin Jakobsson Department of Geological Sciences, Stockholm University, SE-10691 Stockholm, Sweden ([email protected]) Timothy Shank Woods Hole Oceanographic Institution, 266 Woods Hole Road, MS 24, Woods Hole, Massachusetts 02543, USA ([email protected]) [1] We use high-definition seafloor digital imagery and multibeam bathymetric data acquired during the 2007 Arctic Gakkel Vents Expedition (AGAVE) to evaluate the volcanic characteristics of the 85E seg- ment of the ultraslow spreading Gakkel Ridge (9 mm yrÀ1 full rate). Our seafloor imagery reveals that the axial valley is covered by numerous, small-volume (order 1000 m3) lava flows displaying a range of ages and morphologies as well as unconsolidated volcaniclastic deposits with thicknesses up to 10 cm. The valley floor contains two prominent volcanic lineaments made up of axis-parallel ridges and small, cra- tered volcanic cones. -

Quaternary Stratigraphy of the Northwind Ridge, Arctic Ocean
Quaternary Stratigraphy of the Northwind Ridge, Arctic Ocean THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Kevin Allen Crawford B.S. Graduate Program in Geological Sciences The Ohio State University 2010 Master's Examination Committee: Peter-Noel Webb, Advisor Leonid Polyak Lawrence A. Krissek Copyright by Kevin A Crawford 2010 Abstract The Arctic Ocean plays an important role in modulating the world‘s climate. Changes in sea-ice albedo and the export of freshwater into the North Atlantic could have serious repercussions to the climate patterns far beyond the Arctic. To understand fully the impacts of the retreating sea-ice cover and the warming Arctic Ocean we need to look into the past for clues. Paleoenvironments of the Arctic Ocean can be reconstructed by using sea-floor sediment constituents, such as paleobiological and mineral components as well as chemical and paleomagnetic parameters. Three cores raised from the Northwind Ridge, north of the Alaskan continental margin, were chosen to investigate sedimentary patterns and related paleoenvironments in the western Arctic Ocean across a time frame from the Holocene to estimated early Pleistocene. These cores show a range of sedimentation rates decreasing from south to north, thus allowing a development of a relatively high-resolution Upper Quaternary stratigraphy at the southern part of the ridge and a lower-resolution, yet longer stratigraphy for its northern part. In addition to this long stratigraphic coverage, the northern core has well-preserved calcareous microfauna, which provides new insights into paleoceanographic environments. -

Insect Life Cycle ���������������������������������� a Reading A–Z Level L Leveled Book Word Count: 607
Insect Life Cycle A Reading A–Z Level L Leveled Book Word Count: 607 Written by Chuck Garofano Visit www.readinga-z.com www.readinga-z.com for thousands of books and materials. Photo Credits: Front cover, back cover, pages 3, 4, 7, 13, 15 (top): © Brand X Pictures; title page, page 11: © Kenneth Keifer/123RF; page 5: © Eric Isselée/ Dreamstime.com; page 6: © Mike Abbey/Visuals Unlimited, Inc.; page 8: © Richard Williams/123RF; page 9: © Oxford Scientific/Peter Arnold; page 10: © Anthony Bannister/Gallo Images/Corbis; page 12: © Dennis Johnson; Papillo/Corbis; page 14: © 123RF; page 15 (bottom): © ArtToday Written by Chuck Garofano Insect Life Cycle Level L Leveled Book Correlation © Learning A–Z LEVEL L Written by Chuck Garofano Fountas & Pinnell K All rights reserved. Reading Recovery 18 www.readinga-z.com www.readinga-z.com DRA 20 Table of Contents Flower beetle Introduction ................ 4 What Are Insects? ............ 6 Egg ....................... 8 Larva ......................10 Pupa ..................... 12 Ladybug Tropical ant Adult ..................... 13 Introduction Nymph . 14 When you were born, your body Index...................... 16 was shaped a lot like it is now. It was smaller, of course, but you had a head, legs, arms, and a torso. When you grow up, your body shape will be about the same. But some baby animals look nothing like the adults they will become. Insect Life Cycle • Level L 3 4 These animals have a different kind What Are Insects? of life cycle. A life cycle is the series There are more than 800,000 of changes an animal goes through different kinds of insects. -

POLYZOA (BRYOZOA) Sheet 107 ORDER CHEILOSTOMATA CYPHONAUTES LARVAE (BY J
CONSEIL INTERNATIONAL POUR L’EXPLORATION DE LA MER Zooplankton POLYZOA (BRYOZOA) Sheet 107 ORDER CHEILOSTOMATA CYPHONAUTES LARVAE (BY J. S. RYLAND) 1965 https://doi.org/10.17895/ices.pub.4948 Larvae found in the eastern North Atlantic c-2- lb ant. A post. lc la 4d 4e f If 12 4a 4c Plate I. Large cyphonautes with clear shells. 1. Membraniporu membranucea; a, lateral view (North Sea, July) ; b, apical view ((aresund, October) ; c, anterior view (as b) ; d, e, shell outlines of young larvae (Plymouth) ; f, g, variation in shell pro- file (North Sea, July). - 4. Electrapilosa (see also Plate 11); a, lateral view (Plymouth, December); b, apical view (Plymouth, January); c, anterior view (as b); d, e, shell outlines of young larvae (Plymouth). a.o., adhesive organ; f, flange; m, adductor muscle; P.o., pvriform organ; s., stomach. Scales = 200 ,U. All drawings except If, g to scale 1. (Id, e, 4d, e, after ATKINS,1955a; lb, c, f, g, 4b, c, from RYLAND,1964; la, 4a, original). -3- 4f 2b 5b - 2a a 0 0 cu - 5a - 6a 6b 3c 7b 3a 3d 7a Plate 11. Small cyphonautes with granular (particle-encrusted) shells. 2. Electra crustulenta; a, lateral view (Plymouth, January); b, apical view (as a). - 3. E. monostachys; a, lateral view (Plymouth, January); b, apical view (as a); c, shell profile of younger larva (Ply- mouth, January) ; d, lateral view (Burnham-on-Crouch, November). - 4. E. pilosa (see also Plate I) ; f-h, young larvae (Burnham-on-Crouch, November). - 5. Conopeum reticulum; a, lateral view (Burnham-on-Crouch, June) ; b, apical view (as a). -

Lists of Larval Worms from Marine Invertebrates of the Pacific Oc Ast of North America Hilda Lei Ching Hydra Enterprises
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UNL | Libraries University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Parasitology, Harold W. Manter Laboratory of Laboratory of Parasitology 1991 Lists of Larval Worms from Marine Invertebrates of the Pacific oC ast of North America Hilda Lei Ching Hydra Enterprises Follow this and additional works at: http://digitalcommons.unl.edu/parasitologyfacpubs Part of the Aquaculture and Fisheries Commons, Biodiversity Commons, Other Animal Sciences Commons, Parasitology Commons, and the Zoology Commons Ching, Hilda Lei, "Lists of Larval Worms from Marine Invertebrates of the Pacific oC ast of North America" (1991). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 771. http://digitalcommons.unl.edu/parasitologyfacpubs/771 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Ching, Journal of the Helminthological Society of Washington (1991) 58(1). Copyright 1991, HELMSOC. Used by permission. J lIelminthol. Soc. Wash. 5'8(1). \ 991, pp. 57~8 Lists of Larval Worms from Marine Invertebrates of the Pacific Coast of North America HILDA LEI CHING Hydra Enterprises Ltd., P.O. Box 2184, Vancouver, British Columbia, Canada V6B 3V7 ABS"fRAcr: Immature stages of 73 digenetic trematodes are listed by their families, marine invertebrate hosts, and localities and then cross listed according to their molluscan hosts.