Photodegradation of Pesticides and Application of Bioanalytical Methods for Their Detection*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurotoxicity in Preclinical Models of Occupational Exposure to Organophosphorus Compounds
CORE Metadata, citation and similar papers at core.ac.uk Provided by Frontiers - Publisher Connector REVIEW published: 18 January 2017 doi: 10.3389/fnins.2016.00590 Neurotoxicity in Preclinical Models of Occupational Exposure to Organophosphorus Compounds Jaymie R. Voorhees 1, 2*, Diane S. Rohlman 2, 3, Pamela J. Lein 4 and Andrew A. Pieper 1, 2, 5, 6, 7, 8, 9* 1 Department of Psychiatry, University of Iowa Carver College of Medicine, Iowa City, IA, USA, 2 Interdisciplinary Graduate Program in Human Toxicology, University of Iowa Carver College of Medicine, Iowa City, IA, USA, 3 Department of Occupational and Environmental Health, University of Iowa College of Public Health, Iowa City, IA, USA, 4 Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis, Davis, CA, USA, 5 Department of Neurology, University of Iowa Carver College of Medicine, Iowa City, IA, USA, 6 Department of Free Radical and Radiation Biology Program, University of Iowa Carver College of Medicine, Iowa City, IA, USA, 7 Department of Radiation Oncology Holden Comprehensive Cancer Center, University of Iowa Carver College of Medicine, Iowa City, IA, USA, 8 Department of Veteran Affairs, University of Iowa Carver College of Medicine, Iowa City, IA, USA, 9 Weill Cornell Autism Research Program, Weill Cornell Medical College, New York, NY, USA Organophosphorus (OPs) compounds are widely used as insecticides, plasticizers, and fuel additives. These compounds potently inhibit acetylcholinesterase (AChE), the enzyme that inactivates acetylcholine at neuronal synapses, and acute exposure to high Edited by: OP levels can cause cholinergic crisis in humans and animals. Evidence further suggests Stefano L. -

Lifetime Organophosphorous Insecticide Use Among Private Pesticide Applicators in the Agricultural Health Study
Journal of Exposure Science and Environmental Epidemiology (2012) 22, 584 -- 592 & 2012 Nature America, Inc. All rights reserved 1559-0631/12 www.nature.com/jes ORIGINAL ARTICLE Lifetime organophosphorous insecticide use among private pesticide applicators in the Agricultural Health Study Jane A. Hoppin1, Stuart Long2, David M. Umbach3, Jay H. Lubin4, Sarah E. Starks5, Fred Gerr5, Kent Thomas6, Cynthia J. Hines7, Scott Weichenthal8, Freya Kamel1, Stella Koutros9, Michael Alavanja9, Laura E. Beane Freeman9 and Dale P. Sandler1 Organophosphorous insecticides (OPs) are the most commonly used insecticides in US agriculture, but little information is available regarding specific OP use by individual farmers. We describe OP use for licensed private pesticide applicators from Iowa and North Carolina in the Agricultural Health Study (AHS) using lifetime pesticide use data from 701 randomly selected male participants collected at three time periods. Of 27 OPs studied, 20 were used by 41%. Overall, 95% had ever applied at least one OP. The median number of different OPs used was 4 (maximum ¼ 13). Malathion was the most commonly used OP (74%) followed by chlorpyrifos (54%). OP use declined over time. At the first interview (1993--1997), 68% of participants had applied OPs in the past year; by the last interview (2005--2007), only 42% had. Similarly, median annual application days of OPs declined from 13.5 to 6 days. Although OP use was common, the specific OPs used varied by state, time period, and individual. Much of the variability in OP use was associated with the choice of OP, rather than the frequency or duration of application. -

Malathion Human Health and Ecological Risk Assessment Final Report
SERA TR-052-02-02c Malathion Human Health and Ecological Risk Assessment Final Report Submitted to: Paul Mistretta, COR USDA/Forest Service, Southern Region 1720 Peachtree RD, NW Atlanta, Georgia 30309 USDA Forest Service Contract: AG-3187-C-06-0010 USDA Forest Order Number: AG-43ZP-D-06-0012 SERA Internal Task No. 52-02 Submitted by: Patrick R. Durkin Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Fax: (315) 637-0445 E-Mail: [email protected] Home Page: www.sera-inc.com May 12, 2008 Table of Contents Table of Contents............................................................................................................................ ii List of Figures................................................................................................................................. v List of Tables ................................................................................................................................. vi List of Appendices ......................................................................................................................... vi List of Attachments........................................................................................................................ vi ACRONYMS, ABBREVIATIONS, AND SYMBOLS ............................................................... vii COMMON UNIT CONVERSIONS AND ABBREVIATIONS.................................................... x CONVERSION OF SCIENTIFIC NOTATION .......................................................................... -

Biodetoxification of Coumaphos Insecticide Using Immobilized Escherichia Coli Expressing Organophosphorus Hydrolase Enzyme on Cell Surface
Biotechnol. Bioprocess Eng. 2000, 5: 436-440 Biodetoxification of Coumaphos Insecticide Using Immobilized Escherichia coli Expressing Organophosphorus Hydrolase Enzyme on Cell Surface Ayman H. Mansee2, Wilfred Chen1, and Ashok Mulchandani1* 1 Department of Chemical and Environmental Engineering, University of California, Riverside, CA 92521, USA 2 On leave from Pesticide Chemistry Department, Faculty of Agriculture (El-Shatby), Alexandria University, Alexandria, Egypt Abstract Recently, we reported an improved technology for the degradation of organophos- phate nerve agents using whole cells of genetically engineered Escherichia coli that anchored and displayed the enzyme organophosphorus hydrolase on the cell surface. In this paper we report the immobilization of these cells on highly porous sintered glass beads and the subse- quent application of the immobilized cell in a continuous-flow packed bed bioreactor for the biodetoxification of a widely used insecticide, coumaphos. Keywords: OPH, pesticides, nerve agents, bioreactor, porous sintered glass beads INTRODUCTION (APHIS) operates a Tick Eradication Program designed to prevent the re-introduction of Cattle Fever into the Neurotoxic organophosphates (OPs), amongst the United States through ticks (Boophilus microplus and most toxic compound known, are widely used as pesti- Boophilus annulatus) on cattle imported from Mexico. cides, insecticides and chemical warfare agents. In the The primary tool used in the eradication program is a United States annually over 40 million kg of organo- series of dipping vats placed at the border crossing phosphate pesticides are consumed [1] while another 20 points. Cattle coming in from Mexico must be dipped million kg are produced for export [2]. Chlorpyrifos in vats before entering the US. Currently, coumaphos is (lorsban) and parathion are two of most popular pesti- the pesticide of choice for this application. -

Probabilistic Assessment of the Cumulative Acute Exposure to Organophosphorus and Carbamate Insecticides in the Brazilian Diet E.D
Toxicology 222 (2006) 132–142 Probabilistic assessment of the cumulative acute exposure to organophosphorus and carbamate insecticides in the Brazilian diet E.D. Caldas a,∗, P.E. Boon b, J. Tressou c a Department of Pharmaceutical Sciences, College of Health Sciences, University of Bras´ılia, 70919-970 Bras´ılia, DF, Brazil b RIKILT, Institute of Food Safety, Wageningen University and Research Centre, 6708 PD Wageningen, The Netherlands c INRA, Unit´eM´et@risk, Methodologies d’analyse de risque alimentaire INA-PG, 75231 Paris, France Received 10 October 2005; received in revised form 1 February 2006; accepted 13 February 2006 Available online 6 March 2006 Abstract In the present study, the cumulative exposure of 25 acetylcholinesterase (AChE) inhibiting pesticides through the consumption of nine fruits and vegetables by the Brazilian population was assessed. Food consumption data were obtained from a household budget survey conducted in all Brazilian states from July 2002 to June 2003. Residue data from 4001 samples were obtained from the Brazilian national monitoring program on pesticide residues. Relative potency factors (RPF) were calculated with methamidophos or acephate as index compounds (IC), using BMD10 or NOAEL for AChE inhibition, mostly in rat brain, obtained from national and international pesticide evaluations. Monocrotophos and triazophos, in addition to aldicarb, had the highest calculated RPF in any scenario. The exposure to AChE inhibiting pesticides for the general population at P99.9, represented 33.6% of the ARfD as methamidophos and 70.2% ARfD as acephate. The exposure calculated as acephate could exceed the ARfD at the upper bound of the 95% confidence interval for this percentile. -

The Dietary Supplement Specialists
ADVANCED LABORATORIES STYLE GUIDE: LOGO CMYK COLORS CMYK C 100% M o% Y o% K o% THE DIETARY SUPPLEMENT SPECIALISTS GRAY SCALE C 0% M o% Y o% K 50% BLACK/WHITE C 75% M 68% Y 67% K 90% THE DIETARY SUPPLEMENT SPECIALISTS Price and Capabilites list ADVANCED LABORATORIES STYLE GUIDE: LOGO CMYK COLORS CMYK C 100% M o% Y o% K o% THE DIETARY SUPPLEMENT SPECIALISTS ADVANCED LABORATORIES CAN PROVIDE ALL OF YOUR ANALYTICAL TESTING NEEDS: GRAY SCALE • Microbiological Analysis • One-stop Sample Shipping C 0% M o% • Chemical Analysis (HPLC, GC, FTIR, etc.) • Competitive pricing, with value-added at no additional charge. Y o% • Metals Analysis (ICP, ICP/MS) • Quick turn-around time, typically 3-5 business days K 50% • Raw Materials Analysis • Open 6 Days / Week includes receiving deliveries and • Finished Products Analysis reporting results on Saturday. Closed major holidays. • Food Products Analysis • Trend Check™ Online Sample Data • Nutritional Labeling Amino Acids | Botanicals | Microbiological | Metals/Minerals | Nutritional Supplements Nutritional Labeling | Pesticide Screens | Shelf-Life Testing | Vitamins cGMP Compliant - Please come audit us! ISO/IEC 17025:2005 BLACK/WHITE C 75% M 68% Our Trend CheckTM online program allows customers to view Y 67% K 90% all pertinent sample data including final test certificates, up to date test results, the ability to export to Excel for easy data entry or trend analysis, as well as access invoices quickly, efficiently, and securely at any time. All this at no charge! One Day Rush = 100% Surcharge | Two Day Rush = 50% Surcharge | Three Day Rush = 25% Surcharge The information provided by Advanced Laboratories® is based on the most popular testing. -

Investigation on the Behavior of Pesticides in Atmosphere
Aerosol and Air Quality Research, 11: 783–790, 2011 Copyright © Taiwan Association for Aerosol Research ISSN: 1680-8584 print / 2071-1409 online doi: 10.4209/aaqr.2010.10.0085 Investigation on the Behavior of Pesticides in Atmosphere Pasquale Avino1, Giuseppe Cinelli2, Ivan Notardonato2, Mario V. Russo2* 1 DIPIA, INAIL (ex-ISPESL), via Urbana 167, 00184 Rome, Italy 2 Faculty of Agriculture, University of Molise, via De Sanctis, Campobasso, Italy ABSTRACT Although pesticides are widely used in agriculture, they and in particular the relative residues in foodstuffs, water and atmosphere, may cause remarkable sanitary problems due to the harmful effects (carcinogenic and mutagenic effects) on the human health. In fact, their spread in waters and atmosphere can produce undesired effects on various organisms and/or water contamination. This paper shows an analytical approach based on XAD-2 adsorbent and GC analysis for evaluating the pesticide trend in atmosphere: in particular, the pesticides investigated are omethoate, dicrotofos, disulfoton, dimethoate, parathion methyl, formothion, paraoxon ethyl, malaoxon, parathion ethyl, iodofenfos and triazofos. For the analytical methodology a linearity response was obtained (r2 = 0.9988) in GC-NPD whereas the limits of detection range between 2 and 5 pg/μL in GC-NPD with a Relative Standard Deviation below 9.5. Finally, this approach has been successfully applied to real samples: the results show that dimethoate concentration decreases with increasing distance from the sampling site but it is still persistent in atmosphere after few days from the pesticide spraying. Keywords: Pesticides; XAD-2 adsorbent; GC analysis; Atmosphere; Air quality. INTRODUCTION 2009). One of the most important OP reactions is water hydrolysis. -

Redalyc.Methodological Approach for the Trend Evaluation of Pesticides
Revista CENIC. Ciencias Químicas ISSN: 1015-8553 [email protected] Centro Nacional de Investigaciones Científicas Cuba Vincenzo Russo, Mario; Avino, Pasquale; Bisignani, Raffaella Methodological Approach for the Trend Evaluation of Pesticides in Atmosphere: Preliminary Results Revista CENIC. Ciencias Químicas, vol. 36, 2005 Centro Nacional de Investigaciones Científicas La Habana, Cuba Disponible en: http://www.redalyc.org/articulo.oa?id=181620511032 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Revista CENIC Ciencias Químicas, Vol. 36, No. Especial, 2005 Methodological Approach for the Trend Evaluation of Pesticides in Atmosphere: Preliminary Results Mario Vincenzo Russo1*, Pasquale Avino2 and Raffaella Bisignani1 1 Facoltà di Agraria (DISTAAM), Università del Molise, 2 Laboratorio Inquinamento Chimico dell’Aria, Dipartimento Insediamenti Produttivi e Interazione con l’Ambiente – Istituto Superiore per la Prevenzione E la Sicurezza sul Lavoro, Via De Sanctis – 86100 Campobasso (Italy). Ph.: +39-0874-404-634; Fax: +39- 0874-404-652; E-mail: [email protected] Via Urbana 167 – 00184 Rome (Italy). Ph.: +39 064714242, Fax: +39 064744017; E-mail: [email protected] ABSTRACT: Although the pesticides are widely used in agriculture, they, and in particular the relative residues in foodstuff, waters and atmosphere, make a remarkable social alarm and sanitary problems for the harmful effects (carcinogenic and mutagenic effects) producing on the human health. In fact, their spread in waters and atmosphere can produce undesired effects on various organisms and determine the irrigation or drinkable water contamination. -

Recent Advances on Detection of Insecticides Using Optical Sensors
sensors Review Recent Advances on Detection of Insecticides Using Optical Sensors Nurul Illya Muhamad Fauzi 1, Yap Wing Fen 1,2,*, Nur Alia Sheh Omar 1,2 and Hazwani Suhaila Hashim 2 1 Functional Devices Laboratory, Institute of Advanced Technology, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] (N.I.M.F.); [email protected] (N.A.S.O.) 2 Department of Physics, Faculty of Science, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] * Correspondence: [email protected] Abstract: Insecticides are enormously important to industry requirements and market demands in agriculture. Despite their usefulness, these insecticides can pose a dangerous risk to the safety of food, environment and all living things through various mechanisms of action. Concern about the environmental impact of repeated use of insecticides has prompted many researchers to develop rapid, economical, uncomplicated and user-friendly analytical method for the detection of insecticides. In this regards, optical sensors are considered as favorable methods for insecticides analysis because of their special features including rapid detection time, low cost, easy to use and high selectivity and sensitivity. In this review, current progresses of incorporation between recognition elements and optical sensors for insecticide detection are discussed and evaluated well, by categorizing it based on insecticide chemical classes, including the range of detection and limit of detection. Additionally, this review aims to provide powerful insights to researchers for the future development of optical sensors in the detection of insecticides. Citation: Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Hashim, H.S. Recent Keywords: insecticides; optical sensor; recognition element Advances on Detection of Insecticides Using Optical Sensors. -
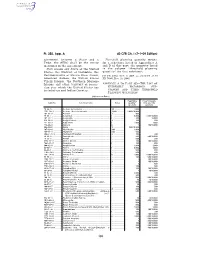
40 CFR Ch. I (7–1–09 Edition) Pt. 355, App. A
Pt. 355, App. A 40 CFR Ch. I (7–1–09 Edition) agreement between a State and a Threshold planning quantity means, Tribe, the SERC shall be the entity for a substance listed in Appendices A identified in the agreement. and B of this part, the quantity listed State means any State of the United in the column ‘‘threshold planning States, the District of Columbia, the quantity’’ for that substance. Commonwealth of Puerto Rico, Guam, [73 FR 65462, Nov. 3, 2008, as amended at 73 American Samoa, the United States FR 76960, Dec. 18, 2008] Virgin Islands, the Northern Mariana Islands, any other territory or posses- APPENDIX A TO PART 355—THE LIST OF sion over which the United States has EXTREMELY HAZARDOUS SUB- jurisdiction and Indian Country. STANCES AND THEIR THRESHOLD PLANNING QUANTITIES [Alphabetical Order] Reportable Threshold plan- CAS No. Chemical name Notes quantity * ning quantity (pounds) (pounds) 75–86–5 ................ Acetone Cyanohydrin ................................................. 10 ................ 1,000 1752–30–3 ............ Acetone Thiosemicarbazide ....................................... 1,000 ........... 1,000/10,000 107–02–8 .............. Acrolein ....................................................................... 1 .................. 500 79–06–1 ................ Acrylamide .................................................................. f ................... 5,000 1,000/10,000 107–13–1 .............. Acrylonitrile ................................................................. f ................... 100 10,000 -

Wednesday Scienti� Ic Session Listings 639–830 Information at a Glance
Chicago | October 17-21 Wednesday Scienti ic Session Listings 639–830 Information at a Glance Important Phone Numbers Annual Meeting Headquarters Office Mercy Hospital Key to Poster Floor by Themes Logistics and Programming 2525 S Michigan Avenue The poster floor begins with Theme A and ends Logistics Chicago, IL 60616 with Theme H. Refer to the poster floor map at McCormick Place: Hall A, (312) 791‑6700 (312) 567‑2000 the end of this booklet. Programming Physicians Immediate Care Theme McCormick Place: Hall A, (312) 791‑6705 811 S. State Street A Development Chicago, IL 60605 B Neural Excitability, Synapses, and Glia: Volunteer Leadership Lounge (312) 566‑9510 Cellular Mechanisms McCormick Place: S505A, (312) 791‑6735 Walgreens Pharmacy C Disorders of the Nervous System General Information Booths (closest to McCormick Place) D Sensory and Motor Systems McCormick Place: 3405 S. Martin Luther King Drive E Integrative Systems: Neuroendocrinology, Gate 3 Lobby, (312) 791‑6724 Chicago, IL 60616 Neuroimmunology and Homeostatic Challenge Hall A (312) 791‑6725 (312) 326‑4064 F Cognition and Behavior Press Offices Venues G Novel Methods and Technology Development Press Room McCormick Place H History, Teaching, Public Awareness, and McCormick Place: Room S501ABC 2301 S. Martin Luther King Drive Societal Impacts in Neuroscience (312) 791‑6730 Chicago, IL 60616 Exhibit Management Fairmont Chicago, Millennium Park Hotel Note: Theme H Posters will be located in Hall A McCormick Place: Hall A, (312) 791‑6740 200 N. Columbus Drive beginning at 1 p.m. on Saturday, Oct. 17, and will Chicago, IL 60601 remain posted until 5 p.m., Sunday, Oct. -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation.