Intensive Blood Pressure Control In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Angiotensin-Converting Enzyme (ACE) Inhibitors Single Entity Agents
Therapeutic Class Overview Angiotensin-Converting Enzyme (ACE) Inhibitors Single Entity Agents Therapeutic Class Overview/Summary: The renin-angiotensin-aldosterone system (RAAS) is the most important component in the homeostatic regulation of blood pressure.1,2 Excessive activity of the RAAS may lead to hypertension and disorders of fluid and electrolyte imbalance.3 Renin catalyzes the conversion of angiotensinogen to angiotensin I. Angiotensin I is then cleaved to angiotensin II by angiotensin- converting enzyme (ACE). Angiotensin II may also be generated through other pathways (angiotensin I convertase).1 Angiotensin II can increase blood pressure by direct vasoconstriction and through actions on the brain and autonomic nervous system.1,3 In addition, angiotensin II stimulates aldosterone synthesis from the adrenal cortex, leading to sodium and water reabsorption. Angiotensin II exerts other detrimental cardiovascular effects including ventricular hypertrophy, remodeling and myocyte apoptosis.1,2 The ACE inhibitors block the conversion of angiotensin I to angiotensin II, and also inhibit the breakdown of bradykinin, a potent vasodilator.4 Evidence-based guidelines recognize the important role that ACE inhibitors play in the treatment of hypertension and other cardiovascular and renal diseases. With the exception of Epaned® (enalapril solution) and Qbrelis® (lisinopril solution), all of the ACE inhibitors are available generically. Table 1. Current Medications Available in Therapeutic Class5-19 Generic Food and Drug Administration -

Interaction of the Sympathetic Nervous System with Other Pressor Systems in Antihypertensive Therapy
Journal of Clinical and Basic Cardiology An Independent International Scientific Journal Journal of Clinical and Basic Cardiology 2001; 4 (3), 185-192 Interaction of the Sympathetic Nervous System with other Pressor Systems in Antihypertensive Therapy Wenzel RR, Baumgart D, Bruck H, Erbel R, Heemann U Mitchell A, Philipp Th, Schaefers RF Homepage: www.kup.at/jcbc Online Data Base Search for Authors and Keywords Indexed in Chemical Abstracts EMBASE/Excerpta Medica Krause & Pachernegg GmbH · VERLAG für MEDIZIN und WIRTSCHAFT · A-3003 Gablitz/Austria FOCUS ON SYMPATHETIC TONE Interaction of SNS J Clin Basic Cardiol 2001; 4: 185 Interaction of the Sympathetic Nervous System with Other Pressor Systems in Antihypertensive Therapy R. R. Wenzel1, H. Bruck1, A. Mitchell1, R. F. Schaefers1, D. Baumgart2, R. Erbel2, U. Heemann1, Th. Philipp1 Regulation of blood pressure homeostasis and cardiac function is importantly regulated by the sympathetic nervous system (SNS) and other pressor systems including the renin-angiotensin system (RAS) and the vascular endothelium. Increases in SNS activity increase mortality in patients with hypertension, coronary artery disease and congestive heart failure. This review summarizes some of the interactions between the main pressor systems, ie, the SNS, the RAS and the vascular endothelium including the endothelin-system. Different classes of cardiovascular drugs interfere differently with the SNS and the other pressor systems. Beta-blockers, ACE-inhibitors and diuretics have no major effect on central SNS activity. Pure vasodilators including nitrates, alpha-blockers and DHP-calcium channel blockers increase SNS activity. In contrast, central sympatholytic drugs including moxonidine re- duce SNS activity. The effects of angiotensin-II receptor antagonist on SNS activity in humans are not clear, experimental data are discussed in this review. -

Effect of Exogenous Glucocorticoid on Osmotically Stimulated Antidiuretic
European Journal of Endocrinology (2006) 155 845–848 ISSN 0804-4643 CLINICAL STUDY Effect of exogenous glucocorticoid on osmotically stimulated antidiuretic hormone secretion and on water reabsorption in man Volker Ba¨hr1, Norma Franzen1, Wolfgang Oelkers2, Andreas F H Pfeiffer1 and Sven Diederich1,2 1Department of Endocrinology, Diabetes and Nutrition, Charite-Universitatsmedizin Berlin, Campus Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany and 2Endokrinologikum Berlin, Centre for Endocrine and Metabolic Diseases, Berlin, Germany (Correspondence should be addressed to V Ba¨hr; Email: [email protected]) Abstract Objective: Glucocorticoids exert tonic suppression of antidiuretic hormone (ADH) secretion. Hypocortisolism in secondary adrenocortical insufficiency can result in a clinical picture similar to the syndrome of inappropriate ADH secretion. On the other hand, in vitro and in vivo results provide evidence for ADH suppression in states of hypercortisolism. To test the hypothesis that ADH suppression is of relevance during glucocorticoid therapy, we investigated the influence of prednisolone on the osmotic stimulation of ADH. Design and methods: Seven healthy men were subjected to water deprivation tests with the measurement of plasma ADH (pADH) and osmolality (posmol) before and after glucocorticoid treatment (5 days 30 mg prednisolone per day). Results: Before glucocorticoid treatment, the volunteers showed a normal test with an adequate increase of pADH (basal 0.54G0.2 to 1.9G0.72 pg/ml (meanGS.D.)) in relation to posmol(basal 283.3G8.5 to 293.7G6 mosmol/kg). After prednisolone intake, pADH was attenuated (!0.4 pg/ml) in spite of an increase of posmol from 289.3G3.6 to 297.0G5.5 mosmol/kg. -

Beta-Blockers for Hypertension: Time to Call a Halt
Journal of Human Hypertension (1998) 12, 807–810 1998 Stockton Press. All rights reserved 0950-9240/98 $12.00 http://www.stockton-press.co.uk/jhh FOR DEBATE Beta-blockers for hypertension: time to call a halt DG Beevers University Department of Medicine, City Hospital, Birmingham B18 7QH, UK Beta-blockers are not very effective at lowering blood the endorsement of beta-blockers by the British Hyper- pressure in elderly hypertensive patients or in Afro- tension Society and other guidelines committees, Caribbeans and these two groups represent a large pro- except possibly for severe resistant hypertension, high portion of people with raised blood pressure. Further- risk post-infarct patients and those with angina pectoris. more they do not prevent more heart attacks than the The time has come to move across to newer, safer, more thiazide diuretics. Beta-blockers can also be dangerous tolerable and more effective antihypertensive agents in many hypertensive patients and even when these whilst continuing to use thiazide diuretics in low doses drugs are not contraindicated, they cause subtle and in the elderly as first choice, providing there are no depressing side effects which should preclude their contraindications. usefulness. The time has come therefore to reconsider Keywords: beta-blockers; hypertension Introduction Safety and tolerability Beta-adrenergic blockers were first introduced in the There is little doubt that the beta-blockers are the early 1960s for the treatment of angina pectoris. most unsafe of all antihypertensive drugs. They can Their antihypertensive properties were not fully precipitate or worsen heart failure in patients with recognised until the celebrated paper by Pritchard myocardial damage and they are contraindicated in and Gillam in 1964.1 They rapidly became popular patients with asthma. -
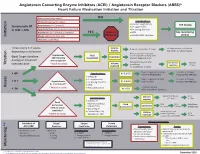
Initiation Titration Assess Monitoring **
Angiotensin Converting Enzyme Inhibitors (ACEI) / Angiotensin Receptor Blockers (ARBS)* Heart Failure Medication Initiation and Titration Bilateral renal artery stenosis NO Moderate/Severe aortic stenosis Considerations: • Baseline cough (ACEi) Hyperkalemia: K+ > 5.2 mmol/L See dosing Symptomatic HF • K+ supplements or LVEF < 40% Renal Dysfunction:Serum creatinine >220 µmol/L • K+ sparing diuretics Hypotension: SBP < 90 mmHg or symptoms YES Refer to • MRA See monitoring Initiation Allergy: angioedema, hives, rash Physician • NSAIDS/COX2 inhibitors section Intolerance: cough (ACEi) Titrate every 1-3 weeks, Volume Reduce/hold diuretic x 2-3 days No improvement, hold/reduce depending on tolerance deplete ACEI/ARB x 1-2 wks & reassess Reassess diuretic dose/other Hypotension Fluid non-essential BP lowering meds Goal: Target dose(see Euvolemic SBP<90mmHg Assessment Consider staggering doses dosing) or maximum with symptoms* Reduce/hold dose of other See Diuretic Reassess Titration * Watch for trends Volume tolerated dose vasodilators algorithm 1-2 wks overload +/- ACEI/ARB x 1-2 weeks Stop K+ supplements, reduce/ Serum K+ in 3-5 days, Considerations: • BP K+ 5.2-5.5 hold MRA (if applicable) Reassess ACEI/ARB dose • Dietary K+ • K+ supplements Stop K+ supplements, MRA. Serum K+ in 2-3 days, • K + Hyperkalemia • K+ sparing diuretics K+ 5.6-6.0 hold ACEI/ARB Reassess ACEI/ARB dose K+ > 5.2 mmol/L* • MRA Assess * Watch for trends • Renal dysfunction Treat hyperkalemia • Scr K+ > 6.0 Refer to MD/NP +/- send to ED Reduce/hold diuretic Scr in Considerations: -

3. Diuretics for Hypertension-A Review and Update
REVIEW Diuretics for Hypertension: A Review and Update George C. Roush1 and Domenic A. Sica2 Downloaded from https://academic.oup.com/ajh/article-abstract/29/10/1130/2622231 by Xenia Agorogianni user on 17 July 2019 This review and update focuses on the clinical features of hydrochlo- ectopy and reduce the risk for sudden cardiac death relative to thi- rothiazide (HCTZ), the thiazide-like agents chlorthalidone (CTDN) and azide-type diuretics used alone. A recent synthesis of 44 trials has indapamide (INDAP), potassium-sparing ENaC inhibitors and aldos- shown that the relative potencies in milligrams among spironolac- terone receptor antagonists, and loop diuretics. Diuretics are the sec- tone (SPIR), amiloride, and eplerenone (EPLER) are approximately ond most commonly prescribed class of antihypertensive medication, from 25 to 10 to 100, respectively, which may be important when SPIR and thiazide-related diuretics have increased at a rate greater than is poorly tolerated. SPIR reduces proteinuria beyond that provided by that of antihypertensive medications as a whole. The latest hyper- other renin angiotensin aldosterone inhibitors. EPLER also reduces tension guidelines have underscored the importance of diuretics for proteinuria and has beneficial effects on endothelial function. While all patients, but particularly for those with salt-sensitive and resist- guidelines often do not differentiate among specific diuretics, this ant hypertension. HCTZ is 4.2–6.2 systolic mm Hg less potent than review demonstrates that these distinctions are important for man- CTDN, angiotensin-converting enzyme inhibitors, beta blockers, and aging hypertension. calcium channel blockers by 24-hour measurements and 5.1 mm Hg systolic less potent than INDAP by office measurements. -

Calcium Channel Blockers
Calcium Channel Blockers Summary In general, calcium channel blockers (CCBs) are used most often for the management of hypertension and angina. There are 2 classes of CCBs: the dihydropyridines (DHPs), which have greater selectivity for vascular smooth muscle cells than for cardiac myocytes, and the non-DHPs, which have greater selectivity for cardiac myocytes and are used for cardiac arrhythmias. The DHPs cause peripheral edema, headaches, and postural hypotension most commonly, all of which are due to the peripheral vasodilatory effects of the drugs in this class of CCBs. The non-DHPs are negative inotropes and chronotropes; they can cause bradycardia and depress AV node conduction, increasing the risk of heart failure exacerbation, bradycardia, and AV block. Clevidipine is a DHP calcium channel blocker administered via continuous IV infusion and used for rapid blood pressure reductions. All CCBs are substrates of CYP3A4, but both diltiazem and verapamil are also inhibitors of 3A4 and have an increased risk of drug interactions. Verapamil also inhibits CYP2C9, CYP2C19, and CYP1A2. Pharmacology CCBs selectively inhibit the voltage-gated L-type calcium channels on cardiac myocytes, vascular smooth muscle cells, and cells within the sinoatrial (SA) and atrioventricular (AV) nodes, preventing influx of extracellular calcium. CCBs act by either deforming the channels, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the major cellular calcium store, the endoplasmic reticulum. Calcium influx via these channels serves for excitation-contraction coupling and electrical discharge in the heart and vasculature. A decrease in intracellular calcium will result in inhibition of the contractile process of the myocardial smooth muscle cells, resulting in dilation of the coronary and peripheral arterial vasculature. -

Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax)
Caffeine and Energy Boosting Drugs: Energy Drinks 800.232.4424 (Voice/TTY) 860.793.9813 (Fax) www.ctclearinghouse.org A Library and Resource Center on Alcohol, Tobacco, Other Drugs, Mental Health and Wellness What are energy drinks? you. You wouldn't use Mountain Dew as a sports Energy drinks are beverages like drink. And a drink like Red Bull and vodka is Red Bull, Venom, Adrenaline Rush, more like strong coffee and whisky than 180, ISO Sprint, and Whoopass, anything else. which contain large doses of caffeine and other legal stimulants What happens when energy drinks are like ephedrine, guarana, and ginseng. combined with alcohol? Energy drinks may contain as much as 80 mg. Energy drinks are also used as mixers with of caffeine, the equivalent of a cup of coffee. alcohol. This combination carries a number of Compared to the 37 mg. of caffeine in a dangers: Mountain Dew, or the 23 mg. in a Coca-Cola Classic, that's a big punch. These drinks are • Since energy drinks are stimulants and marketed to people under 30, especially to alcohol is a depressant, the combination of college students, and are widely available both effects may be dangerous. The stimulant on and off campus. effects can mask how intoxicated you are and prevent you from realizing how much Are there short-term dangers to drinking alcohol you have consumed. Fatigue is one energy drinks? of the ways the body normally tells Individual responses to caffeine vary, and someone that they've had enough to drink. these drinks should be treated carefully • The stimulant effect can give the person the because of how powerful they are. -

Information Presse an Antihypertensive Drug Improves Corticosteroid-Based Skin Treatments
Paris, le 18 mars 2015 Information presse An antihypertensive drug improves corticosteroid-based skin treatments Basic research on blood pressure has led researchers from Inserm (Inserm Unit 1138, “Cordeliers Research Centre”) to obtain unexpected results: drugs used to treat hypertension (high blood pressure) reduce side effects from corticosteroid-based creams used to treat certain skin diseases. This work is published in the Journal of Investigative Dermatology. Corticosteroid-based dermatological creams are indicated for the symptomatic treatment of inflammatory skin conditions, such as atopic dermatitis and psoriasis, for example. However, they have frequent side effects, such as a slight burning sensation, and very often end by inducing skin atrophy (thinning of the skin, which becomes fragile), which is inconvenient for the patient, and for which there is presently no treatment. The researchers from Inserm formulated a hypothesis whereby this harmful effect might be related to the inappropriate activation by these creams of mineralocorticoid receptors located in the epidermis. These receptors, which are present in the kidney, heart, eye, and certain neurons in particular, reacted with aldosterone, a hormone that regulates the blood pressure. Moreover, previous studies also showed them to be highly sensitive to corticosteroids. Application of corticosteroids to cultured skin causes it to become thinner: in 6 days, the thickness of the epidermis was reduced by one-third. The researchers then induced a pharmacological blockade of the receptors by adding specific antagonists to the corticosteroid treatment. The inability of the corticosteroid to bind to the mineralocorticoid receptors restores proliferation of the epidermal cells, and partially corrects epidermal atrophy. From the clinical point of view, it turns out that spironolactone, a drug used for a very long time as an antihypertensive drug (and which has marketing authorisation), is an antagonist of the mineralocorticoid receptor. -

The Necessity and Effectiveness of Mineralocorticoid Receptor Antagonist in the Treatment of Diabetic Nephropathy
Hypertension Research (2015) 38, 367–374 & 2015 The Japanese Society of Hypertension All rights reserved 0916-9636/15 www.nature.com/hr REVIEW The necessity and effectiveness of mineralocorticoid receptor antagonist in the treatment of diabetic nephropathy Atsuhisa Sato Diabetes mellitus is a major cause of chronic kidney disease (CKD), and diabetic nephropathy is the most common primary disease necessitating dialysis treatment in the world including Japan. Major guidelines for treatment of hypertension in Japan, the United States and Europe recommend the use of angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers, which suppress the renin-angiotensin system (RAS), as the antihypertensive drugs of first choice in patients with coexisting diabetes. However, even with the administration of RAS inhibitors, failure to achieve adequate anti-albuminuric, renoprotective effects and a reduction in cardiovascular events has also been reported. Inadequate blockade of aldosterone may be one of the reasons why long-term administration of RAS inhibitors may not be sufficiently effective in patients with diabetic nephropathy. This review focuses on treatment in diabetic nephropathy and discusses the significance of aldosterone blockade. In pre-nephropathy without overt nephropathy, a mineralocorticoid receptor antagonist can be used to enhance the blood pressure-lowering effects of RAS inhibitors, improve insulin resistance and prevent clinical progression of nephropathy. In CKD categories A2 and A3, the addition of a mineralocorticoid receptor antagonist to an RAS inhibitor can help to maintain ‘long-term’ antiproteinuric and anti-albuminuric effects. However, in category G3a and higher, sufficient attention must be paid to hyperkalemia. Mineralocorticoid receptor antagonists are not currently recommended as standard treatment in diabetic nephropathy. -

Report of the Third Working Party of the British Hypertension Society
Journal of Human Hypertension (1999) 13, 569–592 1999 Stockton Press. All rights reserved 0950-9240/99 $15.00 http://www.stockton-press.co.uk/jhh BHS GUIDELINES Guidelines for management of hypertension: report of the third working party of the British Hypertension Society LE Ramsay, B Williams, GD Johnston, GA MacGregor, L Poston, JF Potter, NR Poulter and G Russell for the British Hypertension Society ¼ Use non-pharmacological measures in all hyperten- as first-line therapy for the majority of hypertensive sive and borderline hypertensive people. people. In the absence of compelling indications for ¼ Initiate antihypertensive drug therapy in people with beta-blockade, diuretics or long acting dihydropyrid- sustained systolic blood pressures (BP) у160 mm Hg ine calcium antagonists are preferred to beta-blockers or sustained diastolic BP у100 mm Hg. in older subjects. Compelling indications and contra- ¼ Decide on treatment in people with sustained systolic indications for all antihypertensive drug classes are BP between 140 and 159 mm Hg or sustained diastolic specified. BP between 90 and 99 mm Hg according to the pres- ¼ For most hypertensives, a combination of antihyper- ence or absence of target organ damage, cardio- tensive drugs will be required to achieve the rec- vascular disease or a 10-year coronary heart disease ommended targets for blood pressure control. у (CHD) risk of 15% according to the Joint British ¼ Other drugs that reduce cardiovascular risk must also Societies CHD risk assessment programme/risk chart. be considered. These include aspirin for secondary ¼ In people with diabetes mellitus, initiate antihyperten- у prevention of cardiovascular disease, and primary sive drug therapy if systolic BP is sustained 140 prevention in treated hypertensive subjects over the mm Hg or diastolic BP is sustained у90 mm Hg. -

Study on Cognition and Prognosis in the Elderly (SCOPE)
BLOOD PRESSURE 1999; 8: 177±183 Study on COgnition and Prognosis in the Elderly (SCOPE) L. HANSSON1, H. LITHELL1, I. SKOOG2, F. BARO3,C.M.BA´NKI4, M. BRETELER5, P. U. CARBONIN6, A. CASTAIGNE7, M. CORREIA8, J.-P. DEGAUTE9, D. ELMFELDT10, K. ENGEDAL11, C. FARSANG12, J. FERRO8, V. HACHINSKI13, A. HOFMAN5, O. F. W. JAMES14, E. KRISIN10, M. LEEMAN9, P. W. DE LEEUW15, D. LEYS16, A. LOBO17, G. NORDBY11, B. OLOFSSON10, G. OPOLSKI18, M. PRINCE19, F. M. REISCHIES20, J. B. ROSENFELD21, L. RUILOPE22, J. SALERNO23, R. TILVIS24, P. TRENKWALDER25 AND A. ZANCHETTI26 From 1Uppsala, 2Go¨teborg, Sweden, 3Bierbeek, Belgium, 4Nagyka´llo´, Hungary, 5Rotterdam, The Netherlands, 6Roma, Italy, 7Cre´teil, France, 8Lisboa, Portugal, 9Bruxelles, Belgium, 10Mo¨lndal, Sweden, 11Oslo, Norway, 12Budapest, Hungary, 13London, Ontario, Canada, 14Newcastle upon Tyne, UK, 15Maastricht, The Netherlands, 16Lille, France, 17Zaragoza, Spain, 18Warsaw, Poland, 19London, UK, 20Berlin, Germany, 21Tel-Aviv, Israel, 22Madrid, Spain, 23Washington DC, USA, 24Helsinki, Finland, 25Starnberg, Germany, 26Milan, Italy Hansson L, Lithell H, Skoog I, Baro F, Ba´nki CM, Breteler M, Carbonin PU, Castaigne A, Correia M, Degaute J-P, Elmfeldt D, Engedal K, Farsang C, Ferro J, Hachinski V, Hofman A, James OFW, Krisin E, Leeman M, De Leeuw PW, Leys D, Lobo A, Nordby G, Olofsson B, Opolski G, Prince M, Reischies FM, Rosenfeld JB, Ruilope L, Salerno J, Tilvis R, Trenkwalder P, Zanchetti A. Study on cognition and prognosis in the elderly (SCOPE). Blood Pressure 1999; 8: 177–183. The Study on COgnition and Prognosis in the Elderly (SCOPE) is a multicentre, prospective, randomized, double-blind, parallel-group study designed to compare the effects of candesartan cilexetil and placebo in elderly patients with mild hypertension.