Novel Targets for Interleukin 18 Binding Protein
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DS Human IL-18
® MSD Human IL-18 Kit For quantitative determination in human serum, plasma, and tissue culture supernatants Alzheimer’s Disease IL-18 BioProcess Cardiac Cell Signaling Clinical Immunology Cytokines Growth Factors Hypoxia Immunogenicity Inflammation Metabolic Oncology Interleukin-18 (IL-18) is an 18 kDa cytokine and a co-stimulatory factor that is produced in Kuppfer cells, activated macrophages, Toxicology keratinocytes, and intestinal epithelial cells.1 One of the main functions of IL-18 is to promote the production of IFN-γ from T and NK Vascular cells, particularly in the presence of IL-12p70. IL-18 also promotes the secretion of other proinflammatory cytokines like TNF-α, IL-1β, 2 and GM-CSF that enhance the migration and activtion of neutrophils during microbial infections. IL-18 enhances cytotoxic activity and 2,3 Catalog Numbers proliferation of CD8+ T and NK cells and has been shown to stimulate the production of IL-13 and other Th2 cytokines. Dysregulation of IL-18 may therefore contribute to inflammatory-associated disorders, unchecked infections, autoimmune diseases such as 2,3 Human IL-18 Kit rheumatoid arthritis, acute and chronic kidney injury, cancer, and pathogenic conditions related to metabolic syndrome. Kit size The MSD Human IL - 18 assay is available on 96-well 4-spot plates. This datasheet outlines the performance of the assay. 1 plate K151MCD-1 5 plates K151MCD-2 Assay Sensitivity 25 plates K151MCD-4 IL-18 LLOD (pg/mL) 0.71 The lower limit of detection (LLOD) is a calculated concentration based on a signal Ordering information 2.5 standard deviations above the background (zero calibrator blank). -

Role of Interleukin 36Γ in Host Defense Against Tuberculosis Fadhil Ahsan,1,2,A Pedro Moura-Alves,1,A Ute Guhlich-Bornhof,1 Marion Klemm,1 Stefan H
The Journal of Infectious Diseases MAJOR ARTICLE Role of Interleukin 36γ in Host Defense Against Tuberculosis Fadhil Ahsan,1,2,a Pedro Moura-Alves,1,a Ute Guhlich-Bornhof,1 Marion Klemm,1 Stefan H. E. Kaufmann,1 and Jeroen Maertzdorf1 Downloaded from https://academic.oup.com/jid/article-abstract/214/3/464/2577344 by Deutsches Rheumaforschungs Zentrum user on 26 June 2019 1Department of Immunology, Max Planck Institute for Infection Biology, and 2ZIBI Graduate School Berlin, Germany Tuberculosis remains a major killer worldwide, not the least because of our incomplete knowledge of protective and pathogenic immune mechanism. The roles of the interleukin 1 (IL-1) and interleukin 18 pathways in host defense are well established, as are their regulation through the inflammasome complex. In contrast, the regulation of interleukin 36γ (IL-36γ), a recently described member of the IL-1 family, and its immunological relevance in host defense remain largely unknown. Here we show that Myco- bacterium tuberculosis infection of macrophages induces IL-36γ production in a 2-stage-regulated fashion. In the first stage, mi- crobial ligands trigger host Toll-like receptor and MyD88-dependent pathways, leading to IL-36γ secretion. In the second stage, endogenous IL-1β and interleukin 18 further amplify IL-36γ synthesis. The relevance of this cytokine in the control of M. tuber- culosis is demonstrated by IL-36γ–induced antimicrobial peptides and IL-36 receptor–dependent restriction of M. tuberculosis growth. Thus, we provide first insight into the induction and regulation of the proinflammatory cytokine IL-36γ during tuberculosis. Keywords. IL-36γ; Mycobacterium tuberculosis; TLR; inflammasome; antimicrobial peptide. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

The Role of Interleukin-18 in the Metabolic Syndrome Marius Trøseid1*, Ingebjørg Seljeflot1,2, Harald Arnesen1,2
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Trøseid et al. Cardiovascular Diabetology 2010, 9:11 CARDIO http://www.cardiab.com/content/9/1/11 VASCULAR DIABETOLOGY REVIEW Open Access The role of interleukin-18 in the metabolic syndrome Marius Trøseid1*, Ingebjørg Seljeflot1,2, Harald Arnesen1,2 Abstract The metabolic syndrome is thought to be associated with a chronic low-grade inflammation, and a growing body of evidence suggests that interleukin-18 (IL-18) might be closely related to the metabolic syndrome and its conse- quences. Circulating levels of IL-18 have been reported to be elevated in subjects with the metabolic syndrome, to be closely associated with the components of the syndrome, to predict cardiovascular events and mortality in populations with the metabolic syndrome and to precede the development of type 2 diabetes. IL-18 is found in the unstable atherosclerotic plaque, in adipose tissue and in muscle tissue, and is subject to several regulatory steps including cleavage by caspase-1, inactivation by IL-18 binding protein and the influence of other cytokines in modulating its interaction with the IL-18 receptor. The purpose of this review is to outline the role of IL-18 in the metabolic syndrome, with particular emphasis on cardiovascular risk and the potential effect of life style interventions. Introduction developing CVD is approximately doubled in the meta- The metabolic syndrome is a cluster of risk factors that bolic syndrome [10]. In a meta-analysis including identifies a population with increased risk for developing 43 cohorts, the relative risk for cardiovascular events type 2 diabetes mellitus and cardiovascular disease and death was 1.78, with the highest risk in women [11]. -

Inflammation-Dependent IL18 Signaling Restricts Hepatocellular Carcinoma Growth by Enhancing the Accumulation and Activity of Tumor-Infiltrating Lymphocytes
Published OnlineFirst February 18, 2016; DOI: 10.1158/0008-5472.CAN-15-1548 Cancer Tumor and Stem Cell Biology Research Inflammation-Dependent IL18 Signaling Restricts Hepatocellular Carcinoma Growth by Enhancing the Accumulation and Activity of Tumor- Infiltrating Lymphocytes Geoffrey J. Markowitz1, Pengyuan Yang1,2,3, Jing Fu3, Gregory A. Michelotti4, Rui Chen1, Jianhua Sui5, Bin Yang2, Wen-Hao Qin3, Zheng Zhang6, Fu-Sheng Wang6, Anna Mae Diehl4, Qi-Jing Li7, Hongyang Wang3, and Xiao-Fan Wang1 Abstract Chronic inflammation in liver tissue is an underlying cause of IL18R1 deletion increased tumor burden. Mechanistically, we hepatocellular carcinoma. High levels of inflammatory cytokine foundthatIL18exertedinflammation-dependent tumor-sup- IL18 in the circulation of patients with hepatocellular carcinoma pressive effects largely by promoting the differentiation, activ- correlates with poor prognosis. However, conflicting results have ity, and survival of tumor-infiltrating T cells. Finally, differences been reported for IL18 in hepatocellular carcinoma development in the expression of IL18 in tumor tissue versus nontumor and progression. In this study, we used tissue specimens from tissueweremorepredictiveofpatientoutcomethanoverall hepatocellular carcinoma patients and clinically relevant mouse tissue expression. Taken together, our findings resolve a long- models of hepatocellular carcinoma to evaluate IL18 expression standing contradiction regarding a tumor-suppressive role for and function. In a mouse model of liver fibrosis that recapitulates IL18 in established hepatocellular carcinoma and provide a a tumor-promoting microenvironment, global deletion of the mechanistic explanation for the complex relationship between IL18 receptor IL18R1 enhanced tumor growth and burden. Sim- its expression pattern and hepatocellular carcinoma prognosis. ilarly, in a carcinogen-induced model of liver tumorigenesis, Cancer Res; 76(8); 1–12. -
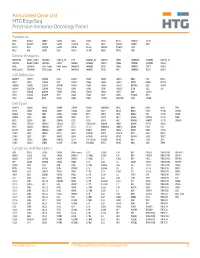
Annotated Gene List HTG Edgeseq Precision Immuno-Oncology Panel
Annotated Gene List HTG EdgeSeq Precision Immuno-Oncology Panel Apoptosis APAF1 BCL2L1 CARD11 CASP4 CD5L FADD KSR2 OPTN SAMD12 TCF19 BAX BCL2L11 CASP1 CASP5 CORO1A FAS LRG1 PLA2G6 SAMD9 XAF1 BCL10 BCL6 CASP10 CASP8 DAPK2 FASLG MECOM PYCARD SPOP BCL2 BID CASP3 CAV1 DAPL1 GLIPR1 MELK RIPK2 TBK1 Cancer Antigens ANKRD30A BAGE2_BAGE3 CEACAM6 CTAG1A_1B LIPE MAGEA3_A6 MAGEC2 PAGE3 SPANXACD SPANXN4 XAGE1B_1E ARMCX6 BAGE4_BAGE5 CEACAM8 CTAG2 MAGEA1 MAGEA4 MTFR2 PAGE4 SPANXB1 SPANXN5 XAGE2 BAGE CEACAM1 CT45_family GAGE_family MAGEA10 MAGEB2 PAGE1 PAGE5 SPANXN1 SYCP1 XAGE3 BAGE_family CEACAM5 CT47_family HPN MAGEA12 MAGEC1 PAGE2 PBK SPANXN3 TEX14 XAGE5 Cell Adhesion ADAM17 CDH15 CLEC5A DSG3 ICAM2 ITGA5 ITGB2 LAMC3 MBL2 PVR UPK2 ADD2 CDH5 CLEC6A DST ICAM3 ITGA6 ITGB3 LAMP1 MTDH RRAS2 UPK3A ADGRE5 CLDN3 CLEC7A EPCAM ICAM4 ITGAE ITGB4 LGALS1 NECTIN2 SELE VCAM1 ALCAM CLEC12A CLEC9A FBLN1 ITGA1 ITGAL ITGB7 LGALS3 OCLN SELL ZYX CD63 CLEC2B DIAPH3 FXYD5 ITGA2 ITGAM ITLN2 LYVE1 OLR1 SELPLG CD99 CLEC4A DLGAP5 IBSP ITGA3 ITGAX JAML M6PR PECAM1 THY1 CDH1 CLEC4C DSC3 ICAM1 ITGA4 ITGB1 L1CAM MADCAM1 PKP1 UNC5D Cell Cycle ANAPC1 CCND3 CDCA5 CENPH CNNM1 ESCO2 HORMAD2 KIF2C MELK ORC6 SKA3 TPX2 ASPM CCNE1 CDCA8 CENPI CNTLN ESPL1 IKZF1 KIF4A MND1 PATZ1 SP100 TRIP13 AURKA CCNE2 CDK1 CENPL CNTLN ETS1 IKZF2 KIF5C MYBL2 PIF1 SP110 TROAP AURKB CCNF CDK4 CENPU DBF4 ETS2 IKZF3 KIFC1 NCAPG PIMREG SPC24 TUBB BEX1 CDC20 CDK6 CENPW E2F2 EZH2 IKZF4 KNL1 NCAPG2 PKMYT1 SPC25 ZWILCH BEX2 CDC25A CDKN1A CEP250 E2F7 GADD45GIP1 KDM5B LMNA NCAPH POC1A SPDL1 BUB1 CDC25C CDKN1B CEP55 ECT2 -

Development of Autoimmune Hair Loss Disease Alopecia Areata Is Associated with Cardiac Dysfunction in C3H/ Hej Mice
Development of Autoimmune Hair Loss Disease Alopecia Areata Is Associated with Cardiac Dysfunction in C3H/ HeJ Mice Eddy Wang1, Katy Chong2, Mei Yu1, Noushin Akhoundsadegh1, David J. Granville3, Jerry Shapiro4, Kevin J. McElwee1* 1 Department of Dermatology and Skin Science, University of British Columbia, Vancouver, BC, Canada, 2 University of British Columbia, Vancouver, BC, Canada, 3 Department of Pathology and Laboratory Medicine, James Hogg Research Centre, Institute for Heart and Lung Health, University of British Columbia, Vancouver, BC, Canada, 4 Department of Dermatology and Skin Science, Vancouver General Hospital, Vancouver, BC, Canada Abstract Alopecia areata (AA) is a chronic autoimmune hair loss disease that affects several million men, women and children worldwide. Previous studies have suggested a link between autoimmunity, stress hormones, and increased cardiovascular disease risk. In the current study, histology, immunohistology, quantitative PCR (qPCR) and ELISAs were used to assess heart health in the C3H/HeJ mouse model for AA and heart tissue response to adrenocorticotropic hormone (ACTH) exposure. Mice with AA exhibited both atrial and ventricular hypertrophy, and increased collagen deposition compared to normal- haired littermates. QPCR revealed significant increases in Il18 (4.6-fold), IL18 receptor-1 (Il18r1; 2.8-fold) and IL18 binding protein (Il18bp; 5.2-fold) in AA hearts. Time course studies revealed a trend towards decreased Il18 in acute AA compared to controls while Il18r1, Il18bp and Casp1 showed similar trends to those of chronic AA affected mice. Immunohistochemistry showed localization of IL18 in chronic AA mouse atria. ELISA indicated cardiac troponin-I (cTnI) was elevated in the serum and significantly increased in AA heart tissue. -

IL-33 Can Promote Survival, Adhesion and Cytokine Production in Human
Laboratory Investigation (2007) 87, 971–978 & 2007 USCAP, Inc All rights reserved 0023-6837/07 $30.00 IL-33 can promote survival, adhesion and cytokine production in human mast cells Motoyasu Iikura1,2, Hajime Suto1,3, Naoki Kajiwara4, Keisuke Oboki5, Tatsukuni Ohno5, Yoshimichi Okayama4, Hirohisa Saito3,5, Stephen J Galli1 and Susumu Nakae1,3,5 IL-33 is a recently identified member of the IL-1 family of molecules, which also includes IL-1 and IL-18. IL-33 binds to the receptor, T1/ST2/IL-1R4, and can promote cytokine secretion by Th2 cells and NF-kB phosphorylation in mouse mast cells. However, the effects of these molecules, especially IL-33, in human mast cells are poorly understood. Expression of the receptors for IL-1 family molecules, specifically, IL-1R1, IL-18R and T1/ST2, was detectable intracellularly in human umbilical cord blood-derived mast cells (HUCBMCs) by flow cytometry, but was scarcely detectable on the cells’ surface. However, IL-1b, IL-18 or IL-33 induced phosphorylation of Erk, p38 and JNK in naı¨ve HUCBMCs, and IL-33 or IL-1b, but not IL-18, enhanced the survival of naive HUCBMCs and promoted their adhesion to fibronectin. IL-33 or IL-1b also induced IL-8 and IL-13 production in naı¨ve HUCBMCs, and enhanced production of these cytokines in IgE/anti-IgE-stimulated HUCBMCs, without enhancing secretion of either PGD2 or histamine. Moreover, IL-33-mediated IL-8 production by HUCBMCs was markedly reduced by the p38 MAPK inhibitor, SB203580. In contrast to findings with mouse mast cells, IL-18 neither induced nor enhanced secretion of the mediators PGD2 or histamine by HUCBMCs. -

Interleukin-18 in Health and Disease
International Journal of Molecular Sciences Review Interleukin-18 in Health and Disease Koubun Yasuda 1 , Kenji Nakanishi 1,* and Hiroko Tsutsui 2 1 Department of Immunology, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan; [email protected] 2 Department of Surgery, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-798-45-6573 Received: 21 December 2018; Accepted: 29 January 2019; Published: 2 February 2019 Abstract: Interleukin (IL)-18 was originally discovered as a factor that enhanced IFN-γ production from anti-CD3-stimulated Th1 cells, especially in the presence of IL-12. Upon stimulation with Ag plus IL-12, naïve T cells develop into IL-18 receptor (IL-18R) expressing Th1 cells, which increase IFN-γ production in response to IL-18 stimulation. Therefore, IL-12 is a commitment factor that induces the development of Th1 cells. In contrast, IL-18 is a proinflammatory cytokine that facilitates type 1 responses. However, IL-18 without IL-12 but with IL-2, stimulates NK cells, CD4+ NKT cells, and established Th1 cells, to produce IL-3, IL-9, and IL-13. Furthermore, together with IL-3, IL-18 stimulates mast cells and basophils to produce IL-4, IL-13, and chemical mediators such as histamine. Therefore, IL-18 is a cytokine that stimulates various cell types and has pleiotropic functions. IL-18 is a member of the IL-1 family of cytokines. IL-18 demonstrates a unique function by binding to a specific receptor expressed on various types of cells. -

Comprehensive Association Study of Genetic Variants in the IL-1 Gene Family in Systemic Juvenile Idiopathic Arthritis
Genes and Immunity (2008) 9, 349–357 & 2008 Nature Publishing Group All rights reserved 1466-4879/08 $30.00 www.nature.com/gene ORIGINAL ARTICLE Comprehensive association study of genetic variants in the IL-1 gene family in systemic juvenile idiopathic arthritis CJW Stock1, EM Ogilvie1, JM Samuel1, M Fife1, CM Lewis2 and P Woo1 1Centre for Paediatric and Adolescent Rheumatology, Windeyer Institute for Medical Sciences, University College London, London, UK and 2Guy’s, Kings and St Thomas’ School of Medicine, London, UK Patients with systemic juvenile idiopathic arthritis (sJIA) have a characteristic daily spiking fever and elevated levels of inflammatory cytokines. Members of the interleukin-1 (IL-1) gene family have been implicated in various inflammatory and autoimmune diseases, and treatment with the IL-1 receptor antagonist, Anakinra, shows remarkable improvement in some patients. This work describes the most comprehensive investigation to date of the involvement of the IL-1 gene family in sJIA. A two-stage case–control association study was performed to investigate the two clusters of IL-1 family genes using a tagging single nucleotide polymorphism (SNP) approach. Genotyping data of 130 sJIA patients and 151 controls from stage 1 highlighted eight SNPs in the IL1 ligand cluster region and two SNPs in the IL1 receptor cluster region as showing a significant frequency difference between the populations. These 10 SNPs were typed in an additional 105 sJIA patients and 184 controls in stage 2. Meta-analysis of the genotypes from both stages showed that three IL1 ligand cluster SNPs (rs6712572, rs2071374 and rs1688075) and one IL1 receptor cluster SNP (rs12712122) show evidence of significant association with sJIA. -

Evolutionary Divergence and Functions of the Human Interleukin (IL) Gene Family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W
UPDATE ON GENE COMPLETIONS AND ANNOTATIONS Evolutionary divergence and functions of the human interleukin (IL) gene family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W. Nebert3* and Vasilis Vasiliou1 1Molecular Toxicology and Environmental Health Sciences Program, Department of Pharmaceutical Sciences, University of Colorado Denver, Aurora, CO 80045, USA 2Department of Clinical Pharmacy, University of Colorado Denver, Aurora, CO 80045, USA 3Department of Environmental Health and Center for Environmental Genetics (CEG), University of Cincinnati Medical Center, Cincinnati, OH 45267–0056, USA *Correspondence to: Tel: þ1 513 821 4664; Fax: þ1 513 558 0925; E-mail: [email protected]; [email protected] Date received (in revised form): 22nd September 2010 Abstract Cytokines play a very important role in nearly all aspects of inflammation and immunity. The term ‘interleukin’ (IL) has been used to describe a group of cytokines with complex immunomodulatory functions — including cell proliferation, maturation, migration and adhesion. These cytokines also play an important role in immune cell differentiation and activation. Determining the exact function of a particular cytokine is complicated by the influence of the producing cell type, the responding cell type and the phase of the immune response. ILs can also have pro- and anti-inflammatory effects, further complicating their characterisation. These molecules are under constant pressure to evolve due to continual competition between the host’s immune system and infecting organisms; as such, ILs have undergone significant evolution. This has resulted in little amino acid conservation between orthologous proteins, which further complicates the gene family organisation. Within the literature there are a number of overlapping nomenclature and classification systems derived from biological function, receptor-binding properties and originating cell type. -

Interleukin-18 and Interleukin-18 Receptor-A Expression in Allergic Asthma
Interleukin-18 and interleukin-18 receptor-a expression in allergic asthma To the Editors: were centrifuged at 4056g (1,500 rpm) for 10 min at 4uC. The supernatant was aspirated and the cells were fixed with The inflammatory process in allergic asthma is initiated by T- 350 mL of 1% paraformaldehyde. Stained cells were then helper (Th) type-2 cells, which produce a repertoire of stored in the dark at 4uC until flow-cytometric acquisition cytokines, including interleukin (IL)-4, IL-5, IL-9 and IL-13, ,48 h after staining. which are necessary for immunoglobulin (Ig)E production, airway eosinophilia and goblet cell hyperplasia [1]. IL-18 is Cells were acquired with a 15-colour LSR II flow cytometer another pro-inflammatory cytokine, initially described as equipped with three lasers (Becton Dickinson Instrument interferon (IFN)-c-inducing factor [2]. IL-18 can act as a Systems, Franklin Lakes, NJ, USA) using the FACSDiva cofactor for Th2 cell development and IgE production [3]. software (BD Biosciences). A lymphocyte gate was set up Recently, an IL-18 gene polymorphism was reported to be around the lymphocyte cell population on the side scatter (SSC) associated with asthma severity and higher serum IL-18 levels: versus CD45 plot and then transferred to a SSC versus CD3 plot to the rs5744247 variant, which has higher transcriptional activity identify CD3-positive and -negative cell populations. CD3- than the wildtype allele [4]. In addition, the IL-18 receptor (IL- positive cell populations were separated from CD4- and CD8- 18R) gene (on 2q21) has been identified as a candidate gene positive cell populations by CD4 versus CD8 plot.