Inflammation-Dependent IL18 Signaling Restricts Hepatocellular Carcinoma Growth by Enhancing the Accumulation and Activity of Tumor-Infiltrating Lymphocytes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Interleukin (IL)17A, F and AF in Inflammation: a Study in Collageninduced Arthritis and Rheumatoid Arthritis
bs_bs_banner Clinical and Experimental Immunology ORIGINAL ARTICLE doi:10.1111/cei.12376 Interleukin (IL)-17A, F and AF in inflammation: a study in collagen-induced arthritis and rheumatoid arthritis S. Sarkar,* S. Justa,* M. Brucks,* Summary J. Endres,† D. A. Fox,† X. Zhou,* Interleukin (IL)-17 plays a critical role in inflammation. Most studies to date F. Alnaimat,* B. Whitaker,‡ have elucidated the inflammatory role of IL-17A, often referred to as IL-17. J. C. Wheeler,‡ B. H. Jones§ and IL-17F is a member of the IL-17 family bearing 50% homology to IL-17A S. R. Bommireddy* and can also be present as heterodimer IL-17AF. This study elucidates the *Section of Rheumatology, Department of Medicine, and the Arizona Arthritis Center, distribution and contribution of IL-17A, F and AF in inflammatory arthritis. University of Arizona, Tucson, AZ, †Divison of Neutralizing antibody to IL-17A alone or IL-17F alone or in combination Rheumatology, Department of Internal Medicine, was utilized in the mouse collagen-induced arthritis (CIA) model to eluci- University of Michigan, Ann Arbor, MI, and date the contribution of each subtype in mediating inflammation. IL-17A, F ‡Biologics Research and §Immunology Discovery and AF were all increased during inflammatory arthritis. Neutralization of Research, Janssen Research and Development, IL-17A reduced the severity of arthritis, neutralization of IL-17A+IL-17F had Spring House, PA, USA the same effect as neutralizing IL-17A, while neutralization of IL-17F had no effect. Moreover, significantly higher levels of IL-17A and IL-17F were detected in peripheral blood mononuclear cells (PBMC) from patients with rheumatoid arthritis (RA) in comparison to patients with osteoarthritis (OA). -

Interleukin-33 Signaling Exacerbates Experimental Infectious Colitis by Enhancing Gut Permeability and Inhibiting Protective Th17 Immunity
www.nature.com/mi ARTICLE OPEN Interleukin-33 signaling exacerbates experimental infectious colitis by enhancing gut permeability and inhibiting protective Th17 immunity Vittoria Palmieri1, Jana-Fabienne Ebel1, Nhi Ngo Thi Phuong1, Robert Klopfleisch2, Vivian Pham Vu3,4, Alexandra Adamczyk1, Julia Zöller1, Christian Riedel5, Jan Buer1, Philippe Krebs3, Wiebke Hansen1, Eva Pastille1 and Astrid M. Westendorf 1 A wide range of microbial pathogens is capable of entering the gastrointestinal tract, causing infectious diarrhea and colitis. A finely tuned balance between different cytokines is necessary to eradicate the microbial threat and to avoid infection complications. The current study identified IL-33 as a critical regulator of the immune response to the enteric pathogen Citrobacter rodentium.We observed that deficiency of the IL-33 signaling pathway attenuates bacterial-induced colitis. Conversely, boosting this pathway strongly aggravates the inflammatory response and makes the mice prone to systemic infection. Mechanistically, IL-33 mediates its detrimental effect by enhancing gut permeability and by limiting the induction of protective T helper 17 cells at the site of infection, thus impairing host defense mechanisms against the enteric pathogen. Importantly, IL-33-treated infected mice supplemented with IL-17A are able to resist the otherwise strong systemic spreading of the pathogen. These findings reveal a novel IL-33/IL-17A crosstalk that controls the pathogenesis of Citrobacter rodentium-driven infectious colitis. Manipulating the dynamics of cytokines may offer new therapeutic strategies to treat specific intestinal infections. 1234567890();,: Mucosal Immunology (2021) 14:923–936; https://doi.org/10.1038/s41385-021-00386-7 INTRODUCTION epithelial cells, and endothelial cells in response to injury6. -
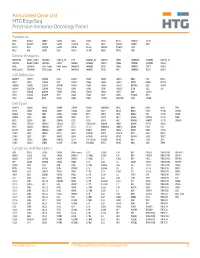
Annotated Gene List HTG Edgeseq Precision Immuno-Oncology Panel
Annotated Gene List HTG EdgeSeq Precision Immuno-Oncology Panel Apoptosis APAF1 BCL2L1 CARD11 CASP4 CD5L FADD KSR2 OPTN SAMD12 TCF19 BAX BCL2L11 CASP1 CASP5 CORO1A FAS LRG1 PLA2G6 SAMD9 XAF1 BCL10 BCL6 CASP10 CASP8 DAPK2 FASLG MECOM PYCARD SPOP BCL2 BID CASP3 CAV1 DAPL1 GLIPR1 MELK RIPK2 TBK1 Cancer Antigens ANKRD30A BAGE2_BAGE3 CEACAM6 CTAG1A_1B LIPE MAGEA3_A6 MAGEC2 PAGE3 SPANXACD SPANXN4 XAGE1B_1E ARMCX6 BAGE4_BAGE5 CEACAM8 CTAG2 MAGEA1 MAGEA4 MTFR2 PAGE4 SPANXB1 SPANXN5 XAGE2 BAGE CEACAM1 CT45_family GAGE_family MAGEA10 MAGEB2 PAGE1 PAGE5 SPANXN1 SYCP1 XAGE3 BAGE_family CEACAM5 CT47_family HPN MAGEA12 MAGEC1 PAGE2 PBK SPANXN3 TEX14 XAGE5 Cell Adhesion ADAM17 CDH15 CLEC5A DSG3 ICAM2 ITGA5 ITGB2 LAMC3 MBL2 PVR UPK2 ADD2 CDH5 CLEC6A DST ICAM3 ITGA6 ITGB3 LAMP1 MTDH RRAS2 UPK3A ADGRE5 CLDN3 CLEC7A EPCAM ICAM4 ITGAE ITGB4 LGALS1 NECTIN2 SELE VCAM1 ALCAM CLEC12A CLEC9A FBLN1 ITGA1 ITGAL ITGB7 LGALS3 OCLN SELL ZYX CD63 CLEC2B DIAPH3 FXYD5 ITGA2 ITGAM ITLN2 LYVE1 OLR1 SELPLG CD99 CLEC4A DLGAP5 IBSP ITGA3 ITGAX JAML M6PR PECAM1 THY1 CDH1 CLEC4C DSC3 ICAM1 ITGA4 ITGB1 L1CAM MADCAM1 PKP1 UNC5D Cell Cycle ANAPC1 CCND3 CDCA5 CENPH CNNM1 ESCO2 HORMAD2 KIF2C MELK ORC6 SKA3 TPX2 ASPM CCNE1 CDCA8 CENPI CNTLN ESPL1 IKZF1 KIF4A MND1 PATZ1 SP100 TRIP13 AURKA CCNE2 CDK1 CENPL CNTLN ETS1 IKZF2 KIF5C MYBL2 PIF1 SP110 TROAP AURKB CCNF CDK4 CENPU DBF4 ETS2 IKZF3 KIFC1 NCAPG PIMREG SPC24 TUBB BEX1 CDC20 CDK6 CENPW E2F2 EZH2 IKZF4 KNL1 NCAPG2 PKMYT1 SPC25 ZWILCH BEX2 CDC25A CDKN1A CEP250 E2F7 GADD45GIP1 KDM5B LMNA NCAPH POC1A SPDL1 BUB1 CDC25C CDKN1B CEP55 ECT2 -

Development of Autoimmune Hair Loss Disease Alopecia Areata Is Associated with Cardiac Dysfunction in C3H/ Hej Mice
Development of Autoimmune Hair Loss Disease Alopecia Areata Is Associated with Cardiac Dysfunction in C3H/ HeJ Mice Eddy Wang1, Katy Chong2, Mei Yu1, Noushin Akhoundsadegh1, David J. Granville3, Jerry Shapiro4, Kevin J. McElwee1* 1 Department of Dermatology and Skin Science, University of British Columbia, Vancouver, BC, Canada, 2 University of British Columbia, Vancouver, BC, Canada, 3 Department of Pathology and Laboratory Medicine, James Hogg Research Centre, Institute for Heart and Lung Health, University of British Columbia, Vancouver, BC, Canada, 4 Department of Dermatology and Skin Science, Vancouver General Hospital, Vancouver, BC, Canada Abstract Alopecia areata (AA) is a chronic autoimmune hair loss disease that affects several million men, women and children worldwide. Previous studies have suggested a link between autoimmunity, stress hormones, and increased cardiovascular disease risk. In the current study, histology, immunohistology, quantitative PCR (qPCR) and ELISAs were used to assess heart health in the C3H/HeJ mouse model for AA and heart tissue response to adrenocorticotropic hormone (ACTH) exposure. Mice with AA exhibited both atrial and ventricular hypertrophy, and increased collagen deposition compared to normal- haired littermates. QPCR revealed significant increases in Il18 (4.6-fold), IL18 receptor-1 (Il18r1; 2.8-fold) and IL18 binding protein (Il18bp; 5.2-fold) in AA hearts. Time course studies revealed a trend towards decreased Il18 in acute AA compared to controls while Il18r1, Il18bp and Casp1 showed similar trends to those of chronic AA affected mice. Immunohistochemistry showed localization of IL18 in chronic AA mouse atria. ELISA indicated cardiac troponin-I (cTnI) was elevated in the serum and significantly increased in AA heart tissue. -

Interleukin-18 in Health and Disease
International Journal of Molecular Sciences Review Interleukin-18 in Health and Disease Koubun Yasuda 1 , Kenji Nakanishi 1,* and Hiroko Tsutsui 2 1 Department of Immunology, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan; [email protected] 2 Department of Surgery, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-798-45-6573 Received: 21 December 2018; Accepted: 29 January 2019; Published: 2 February 2019 Abstract: Interleukin (IL)-18 was originally discovered as a factor that enhanced IFN-γ production from anti-CD3-stimulated Th1 cells, especially in the presence of IL-12. Upon stimulation with Ag plus IL-12, naïve T cells develop into IL-18 receptor (IL-18R) expressing Th1 cells, which increase IFN-γ production in response to IL-18 stimulation. Therefore, IL-12 is a commitment factor that induces the development of Th1 cells. In contrast, IL-18 is a proinflammatory cytokine that facilitates type 1 responses. However, IL-18 without IL-12 but with IL-2, stimulates NK cells, CD4+ NKT cells, and established Th1 cells, to produce IL-3, IL-9, and IL-13. Furthermore, together with IL-3, IL-18 stimulates mast cells and basophils to produce IL-4, IL-13, and chemical mediators such as histamine. Therefore, IL-18 is a cytokine that stimulates various cell types and has pleiotropic functions. IL-18 is a member of the IL-1 family of cytokines. IL-18 demonstrates a unique function by binding to a specific receptor expressed on various types of cells. -

IL-17-Producing Cells in Tumor Immunity: Friends Or Foes?
Immune Netw. 2020 Feb;20(1):e6 https://doi.org/10.4110/in.2020.20.e6 pISSN 1598-2629·eISSN 2092-6685 Review Article IL-17-Producing Cells in Tumor Immunity: Friends or Foes? Da-Sol Kuen 1,2, Byung-Seok Kim 1, Yeonseok Chung 1,2,* 1Laboratory of Immune Regulation, Institute of Pharmaceutical Sciences, Seoul National University, Seoul 08826, Korea 2BK21 Plus Program, Institute of Pharmaceutical Sciences, Seoul National University, Seoul 08826, Korea Received: Dec 29, 2019 Revised: Jan 25, 2020 ABSTRACT Accepted: Jan 26, 2020 IL-17 is produced by RAR-related orphan receptor gamma t (RORγt)-expressing cells *Correspondence to including Th17 cells, subsets of γδT cells and innate lymphoid cells (ILCs). The biological Yeonseok Chung significance of IL-17-producing cells is well-studied in contexts of inflammation, Laboratory of Immune Regulation and BK21 Plus Program, Institute of Pharmaceutical autoimmunity and host defense against infection. While most of available studies in tumor + Sciences, Seoul National University, 1 Gwanak- immunity mainly focused on the role of T-bet-expressing cells, including cytotoxic CD8 ro, Gwanak-gu, Seoul 08826, Korea. T cells and NK cells, and their exhaustion status, the role of IL-17-producing cells remains E-mail: [email protected] poorly understood. While IL-17-producing T-cells were shown to be anti-tumorigenic in Copyright © 2020. The Korean Association of adoptive T-cell therapy settings, mice deficient in type 17 genes suggest a protumorigenic Immunologists potential of IL-17-producing cells. This review discusses the features of IL-17-producing This is an Open Access article distributed cells, of both lymphocytic and myeloid origins, as well as their suggested pro- and/or anti- under the terms of the Creative Commons tumorigenic functions in an organ-dependent context. -

Evolutionary Divergence and Functions of the Human Interleukin (IL) Gene Family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W
UPDATE ON GENE COMPLETIONS AND ANNOTATIONS Evolutionary divergence and functions of the human interleukin (IL) gene family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W. Nebert3* and Vasilis Vasiliou1 1Molecular Toxicology and Environmental Health Sciences Program, Department of Pharmaceutical Sciences, University of Colorado Denver, Aurora, CO 80045, USA 2Department of Clinical Pharmacy, University of Colorado Denver, Aurora, CO 80045, USA 3Department of Environmental Health and Center for Environmental Genetics (CEG), University of Cincinnati Medical Center, Cincinnati, OH 45267–0056, USA *Correspondence to: Tel: þ1 513 821 4664; Fax: þ1 513 558 0925; E-mail: [email protected]; [email protected] Date received (in revised form): 22nd September 2010 Abstract Cytokines play a very important role in nearly all aspects of inflammation and immunity. The term ‘interleukin’ (IL) has been used to describe a group of cytokines with complex immunomodulatory functions — including cell proliferation, maturation, migration and adhesion. These cytokines also play an important role in immune cell differentiation and activation. Determining the exact function of a particular cytokine is complicated by the influence of the producing cell type, the responding cell type and the phase of the immune response. ILs can also have pro- and anti-inflammatory effects, further complicating their characterisation. These molecules are under constant pressure to evolve due to continual competition between the host’s immune system and infecting organisms; as such, ILs have undergone significant evolution. This has resulted in little amino acid conservation between orthologous proteins, which further complicates the gene family organisation. Within the literature there are a number of overlapping nomenclature and classification systems derived from biological function, receptor-binding properties and originating cell type. -

Decrease in Mucosal IL17A, IFN and IL10 Expressions in Active Crohn's
nutrients Article Decrease in Mucosal IL17A, IFNγ and IL10 Expressions in Active Crohn’s Disease Patients Treated with High-Dose Vitamin D Alone or Combined with Infliximab Mia Bendix 1,*, Anders Dige 1, Søren Peter Jørgensen 1, Jens Frederik Dahlerup 1 , Bo Martin Bibby 2, Bent Deleuran 3,4 and Jørgen Agnholt 1 1 Department of Hepatology and Gastroenterology, Aarhus University Hospital, 8200 Aarhus, Denmark; [email protected] (A.D.); [email protected] (S.P.J.); [email protected] (J.F.D.); [email protected] (J.A.) 2 Department of Public Health—Department of Biostatistics, Aarhus University, 8000 Aarhus, Denmark; [email protected] 3 Department of Rheumatology, Aarhus University Hospital, 8200 Aarhus, Denmark; [email protected] 4 Department of Biomedicine, Aarhus University, 8000 Aarhus, Denmark * Correspondence: [email protected] Received: 5 November 2020; Accepted: 26 November 2020; Published: 30 November 2020 Abstract: Background: Vitamin D treatment may reduce Crohn’s disease (CD) activity by modulating the mucosal immune function. We investigated if high-dose vitamin D +/ infliximab modulated − the mucosal cytokine expression in active CD. Methods: Forty CD patients were randomized into: infliximab + vitamin D; infliximab + placebo-vitamin D; placebo-infliximab + vitamin D or placebo-infliximab + placebo-vitamin D. Infliximab (5 mg/kg) and placebo-infliximab were administered at weeks 0, 2 and 6. Oral vitamin D was administered as bolus 200,000 international units (IU) per week 0 followed by 20,000 IU/day for 7 weeks or placebo. Endoscopy with biopsies was performed at weeks 0 and 7 where endoscopic activity was measured and mucosal mRNA cytokine expression was examined. -

Il1f10 (NM 153077) Mouse Tagged ORF Clone – MR223925 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for MR223925 Il1f10 (NM_153077) Mouse Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: Il1f10 (NM_153077) Mouse Tagged ORF Clone Tag: Myc-DDK Symbol: Il1f10 Synonyms: MGC130267; MGC130268 Vector: pCMV6-Entry (PS100001) E. coli Selection: Kanamycin (25 ug/mL) Cell Selection: Neomycin ORF Nucleotide >MR223925 ORF sequence Sequence: Red=Cloning site Blue=ORF Green=Tags(s) TTTTGTAATACGACTCACTATAGGGCGGCCGGGAATTCGTCGACTGGATCCGGTACCGAGGAGATCTGCC GCCGCGATCGCC ATGTGCTCCCTTCCCATGGCAAGATACTACATAATCAAGGATGCACATCAAAAGGCTTTGTACACACGGA ATGGCCAGCTCCTGCTGGGAGACCCTGATTCAGACAATTATAGTCCAGAGAAGGTCTGTATCCTTCCTAA CCGAGGCCTAGACCGCTCCAAGGTCCCCATCTTCCTGGGGATGCAGGGAGGAAGTTGCTGCCTGGCGTGT GTAAAGACAAGAGAGGGACCTCTCCTGCAGCTGGAGGATGTGAACATCGAGGACCTATACAAGGGAGGTG AACAAACCACCCGTTTCACCTTTTTCCAGAGAAGCTTGGGATCTGCCTTCAGGCTTGAGGCTGCTGCCTG CCCTGGCTGGTTTCTCTGTGGCCCAGCTGAGCCCCAGCAGCCAGTGCAGCTCACCAAAGAGAGTGAACCC TCCACCCATACTGAATTCTACTTTGAGATGAGTCGG ACGCGTACGCGGCCGCTCGAGCAGAAACTCATCTCAGAAGAGGATCTGGCAGCAAATGATATCCTGGATT ACAAGGATGACGACGATAAGGTTTAA Protein Sequence: >MR223925 protein sequence Red=Cloning site Green=Tags(s) MCSLPMARYYIIKDAHQKALYTRNGQLLLGDPDSDNYSPEKVCILPNRGLDRSKVPIFLGMQGGSCCLAC VKTREGPLLQLEDVNIEDLYKGGEQTTRFTFFQRSLGSAFRLEAAACPGWFLCGPAEPQQPVQLTKESEP STHTEFYFEMSR TRTRPLEQKLISEEDLAANDILDYKDDDDKV Restriction Sites: SgfI-MluI This product is to -

IL1F10 Monoclonal Antibody
Product Datasheet IL1F10 Monoclonal Antibody Catalog No: #42049 Orders: [email protected] Description Support: [email protected] Product Name IL1F10 Monoclonal Antibody Host Species Mouse Clonality Monoclonal Purification protein G purifed Applications IHC Species Reactivity Hu Specificity specific for Human IL1F10 denatured and native forms Immunogen Type protein Immunogen Description Recombinant Human IL1F10 protein Target Name IL1F10 Other Names IL-1F10, Family of interleukin 1-theta, FIL1 theta Interleukin-1 HY2, IL-1HY2, Interleukin-1 theta, IL-1 theta Interleukin-38, IL-38 Accession No. Swiss-Prot#: Q8WWZ1 Concentration 1.0mg/mL Formulation Preservative: 0.03% Proclin 300 Constituents: 50% Glycerol, 0.01M PBS, PH 7.4 Storage Store at -20°C Application Details Immunohistochemistry: 1:20 - 1:200 Images Immunohistochemical analysis of paraffin-embedded Human Colon cancer tissue using #42049 at dilution of 1:200. Address: 8400 Baltimore Ave. Suite 302 College Park MD 20740 USA http://www.sabbiotech.com 1 Immunohistochemical analysis of paraffin-embedded Human live cancer tissue using #42049 at dilution of 1:200. Immunohistochemical analysis of paraffin-embedded Human spleen tissue using #42049 at dilution of 1:200. Background Cytokine with immunomodulatory activity. Alone, does not induce cytokine production, but reduces IL22 and IL17A production by T-cells in response to heat-killed Candida albicans. Reduces IL36G-induced production of IL8 by peripheral blood mononuclear cells. Increases IL6 production by dendritic cells stimulated by bacterial lipopolysaccharides (LPS). Ligand for IL-36R/IL1RL2. References "IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist." van de Veerdonk F.L., Stoeckman A.K., Wu G., Boeckermann A.N., Azam T., Netea M.G., Joosten L.A., van der Meer J.W., Hao R., Kalabokis V., Dinarello C.A. -

Fibrosis Accumulation in Idiopathic Pulmonary Apoptosis
Increased Cell Surface Fas Expression Is Necessary and Sufficient To Sensitize Lung Fibroblasts to Fas Ligation-Induced Apoptosis: Implications for Fibroblast This information is current as Accumulation in Idiopathic Pulmonary of October 1, 2021. Fibrosis Murry W. Wynes, Benjamin L. Edelman, Amanda G. Kostyk, Michael G. Edwards, Christopher Coldren, Steve D. Groshong, Gregory P. Cosgrove, Elizabeth F. Redente, Alison Bamberg, Kevin K. Brown, Nichole Reisdorph, Downloaded from Rebecca C. Keith, Stephen K. Frankel and David W. H. Riches J Immunol 2011; 187:527-537; Prepublished online 1 June 2011; doi: 10.4049/jimmunol.1100447 http://www.jimmunol.org/ http://www.jimmunol.org/content/187/1/527 Supplementary http://www.jimmunol.org/content/suppl/2011/06/01/jimmunol.110044 Material 7.DC1 References This article cites 62 articles, 18 of which you can access for free at: by guest on October 1, 2021 http://www.jimmunol.org/content/187/1/527.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2011 by The American Association of Immunologists, Inc.