ORIGINAL ARTICLE Mirror of Research in Veterinary Sciences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
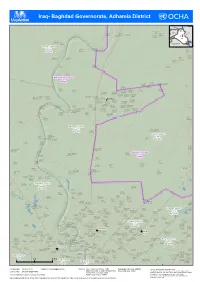
Iraq- Baghdad Governorate, Adhamia District
( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( Iraq- Baghdad Governorate, Adham( ia District ( ( Turkey Mosul! ! Albu Blai'a Erbil village Syria Ahmad al Al-Hajiyah IQ-P12429 Mahmud al Iran ( Mahmud River Al Bu Algah Khamis Baghdad Jadida Qaryat Albu IQ-P09040 IQ-P12203 IQ-P12398 IQ-P12326 Ramadi! ( IQ-P12308 ( ( Tannom ( !\ IQ-P12525 Jordan Najaf! Basrah! Hay Masqat Jurf Al IQ-P12295 Milah Bohroz Mahbubiyah ( SIaQ-uPd12i 2A(32rabia KIQu-wP1a2i3t22 ( IQ-P08417 ( ( ( Mohamed sakran Tarmia District IQ-P12335 Rashdiyah Muhammad (Noor village) Um Al al Jeb اﻟطﺎرﻣﯾﺔ IQ-P08355 Rumman IQ-P08339 ( ( IQ-P12386 IQ-D045 ( Al Halfayah village - 3 Mehdi al Ahmad Jamil Abdul Karim IQ-P12171 Hasan ( ( IQ-P09041 IQ-P08224 ( ( Al A`baseen IQ-P12332 ( village IQ-P12157 ( Sayyid Ahwiz Muhammad Musa Agha IQ-P08231 IQ-P08360 ( ( IQ-P12339 ( ( (( ( ( Fatah Chraiky IQ-P08312 IQ-P08307 ( ( ( Baghdad Governorate ( ( ﺑﻐداد IQ-G07 Qaryat Al Mohamad Khedidan 1 ( Khalaf Tameem Sakran IQ-P12318 ( ( ( Al-Mahmoud IQ-P12349 ( ( Al A'tb(a IQ-P12334 (( IQ-P12313 ( ( Qaryat ( village ( ( Darwesha IQ-P12161 ( Khalaf al IQ-P12359 ( ( Ulwan Muhammad Ja`atah Ali al Hajj ( Hussayniya - al `Abd al `Abbas IQ-P08333 Al Hussayniya IQ-P08297 Mahala 224 IQ-P12385 ( ( ( ( Al Hussainiya (Mahala 222) IQ-P12337 Al Husayniya ( IQ-P08330 ( ( ( - Mahalla 221 - Mahalla 213 IQ-P08264 ( IQ-P08243 IQ-P08251 Al Hussainiya Hussayniya - Al Hussayniya ( ( - Mahalla 216 Mahala 218 - Mahalla 220 IQ-P08253 ( IQ-P08329 IQ-P08261 ( Mahmud al Abdul Aziz Hay Al Waqf Al Hussainiya - ( ( Qal`at Jasim `Ulaywi Hamadi -

The Resurgence of Asa'ib Ahl Al-Haq
December 2012 Sam Wyer MIDDLE EAST SECURITY REPORT 7 THE RESURGENCE OF ASA’IB AHL AL-HAQ Photo Credit: Asa’ib Ahl al-Haq protest in Kadhimiya, Baghdad, September 2012. Photo posted on Twitter by Asa’ib Ahl al-Haq. All rights reserved. Printed in the United States of America. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopy, recording, or any information storage or retrieval system, without permission in writing from the publisher. ©2012 by the Institute for the Study of War. Published in 2012 in the United States of America by the Institute for the Study of War. 1400 16th Street NW, Suite 515 Washington, DC 20036. http://www.understandingwar.org Sam Wyer MIDDLE EAST SECURITY REPORT 7 THE RESURGENCE OF ASA’IB AHL AL-HAQ ABOUT THE AUTHOR Sam Wyer is a Research Analyst at the Institute for the Study of War, where he focuses on Iraqi security and political matters. Prior to joining ISW, he worked as a Research Intern at AEI’s Critical Threats Project where he researched Iraqi Shi’a militia groups and Iranian proxy strategy. He holds a Bachelor’s Degree in Political Science from Middlebury College in Vermont and studied Arabic at Middlebury’s school in Alexandria, Egypt. ABOUT THE INSTITUTE The Institute for the Study of War (ISW) is a non-partisan, non-profit, public policy research organization. ISW advances an informed understanding of military affairs through reliable research, trusted analysis, and innovative education. ISW is committed to improving the nation’s ability to execute military operations and respond to emerging threats in order to achieve U.S. -

Dora – Baghdad – Government Employees – Forced Relocation – Kurdish Areas – Housing
Refugee Review Tribunal AUSTRALIA RRT RESEARCH RESPONSE Research Response Number: IRQ31805 Country: Iraq Date: 22 May 2007 Keywords: Iraq – Kurds – Shia – Dora – Baghdad – Government employees – Forced relocation – Kurdish areas – Housing This response was prepared by the Country Research Section of the Refugee Review Tribunal (RRT) after researching publicly accessible information currently available to the RRT within time constraints. This response is not, and does not purport to be, conclusive as to the merit of any particular claim to refugee status or asylum. Questions 1. Are insurgent/terrorist groups in Iraq targeting anyone who works alongside the pro- coalition forces in the rebuilding of Iraq? 2. Are they targeting Iraqi government workers who perform a co-ordinating role in this regard? 3. Do the insurgents target Shia Iraqis? 4. What is the level of instability within Dora for Kurdish Shiites? 5. Are Kurdish Shiites from Dora being forced to relocate from Dora? 6. Can Kurdish Shiites from Dora safely relocate elsewhere in Baghdad? 7. Can Kurdish Shiites safely relocate to the Kurdish areas of Iraq? 8. Are the local authorities in the Kurdish region imposing regulations to limit the influx of refugees from other parts of Iraq? 9. What has the impact of these internal refugees had on housing and rent in the Kurdish region? RESPONSE 1. Are insurgent/terrorist groups in Iraq targeting anyone who works alongside the pro-coalition forces in the rebuilding of Iraq? 2. Are they targeting Iraqi government workers who perform a co-ordinating -

Towards a Deleuzian Approach in Urban Design
Difference and Repetition in Redevelopment Projects for the Al Kadhimiya Historical Site, Baghdad, Iraq: Towards a Deleuzian Approach in Urban Design A Dissertation submitted to the Graduate School of the University of Cincinnati In partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY IN ARCHITECTURE In the School of Architecture and Interior Design Of the college of Design, Architecture, Art, and Planning 2018 By Najlaa K. Kareem Bachelor of Architecture, University of Technology 1999 Master of Science in Urban and Regional Planning, University of Baghdad 2004 Dissertation Committee: Adrian Parr, PhD (Chair) Laura Jenkins, PhD Patrick Snadon, PhD Abstract In his book Difference and Repetition, the French philosopher Gilles Deleuze distinguishes between two theories of repetition, one associated with the ‘Platonic’ theory and the other with the ‘Nietzschean’ theory. Repetition in the ‘Platonic’ theory, via the criterion of accuracy, can be identified as a repetition of homogeneity, using pre-established similitude or identity to repeat the Same, while repetition in the ‘Nietzschean’ theory, via the criterion of authenticity, is aligned with the virtual rather than real, producing simulacra or phantasms as a repetition of heterogeneity. It is argued in this dissertation that the distinction that Deleuze forms between modes of repetition has a vital role in his innovative approaches to the Nietzschean’s notion of ‘eternal return’ as a differential ontology, offering numerous insights into work on issues of homogeneity and heterogeneity in a design process. Deleuze challenges the assumed capture within a conventional perspective by using German philosopher Friedrich Nietzsche’s conception of the ‘eternal return.’ This dissertation aims to question the conventional praxis of architecture and urban design formalisms through the impulse of ‘becoming’ and ‘non- representational’ thinking of Deleuze. -

The Baghdad Security Plan Begins
A PUBLICATION OF THE INSTITUTE FOR THE STUDY OF WAR AND WEEKLYSTANDARD.COM A PUBLICATION OF THE INSTITUTE FOR THE STUDY OF WAR AND WEEKLYSTANDARD.COM U.S. Army Sgt. Scott Monahan, a tactical human intelligence team leader, collects an entourage of children while on a civil affairs mission in the Rabi area of Adhamiyah, Baghdad, on February 26, 2007. February 10, 2007 – March 5, 2007 Enforcing the Law: The Baghdad Security Plan Begins by KIMBERLY KAGAN This report, the second in a series, describes the purpose, course, and results of Coalition operations in Baghdad during the fi rst three weeks of Operation Enforcing the Law (also known as the Baghdad Security Plan), from General Petraeus’ assumption of command on February 10, 2007, through March 5. It describes the fl ow of American and Iraqi forces into Baghdad; American and Iraqi command relationships; the efforts of those forces to prepare positions and develop intelligence in critical neighborhoods; the limited clearing operations that the forces already in Baghdad have conducted; and operations against the so-called Mahdi army, or Jaysh al Mahdi, in Baghdad. It describes and evaluates the apparent responses of the Jaysh al Mahdi and al Qaeda to these preparations and early operations, and highlights some of the differences between this operation and last year’s offensives in Baghdad, Operations Together Forward I and II. PAGE 1 • FEBRUARY 10, 2007 – MARCH 5, 2007 A PUBLICATION OF THE INSTITUTE FOR THE STUDY OF WAR AND WEEKLYSTANDARD.COM Mission struction missions in Iraq. He requests troops resident Bush announced an increase for Iraq through the United States Central Com- in U.S. -

Iraq Index Tracking Variables of Reconstruction & Security in Post-Saddam Iraq
Iraq Index Tracking Variables of Reconstruction & Security in Post-Saddam Iraq http://www.brookings.edu/iraqindex March 26, 2009 Michael E. O’Hanlon Jason H. Campbell For more information please contact Jason Campbell at [email protected] TABLE OF CONTENTS Tracking the Aftermath of the Surge Page Estimated Number of Iraqi Civilian Fatalities by Month, May 2003-Present…………………………………………………………………………………4 Detailed Explanation of Iraqi Civilian Fatality Estimates by Time Period…………………………………………………………………………………….5 Enemy-Initiated Attacks Against the Coalition and Its Partners, by Week..…………………………………………………………………....………….....6 Iraqi Military and Police Killed since January 2005……………………………………………………………………………………………..………...……6 Current Disposition of U.S./Coalition Forces in Iraq, by Multi-National Division (MND)………………………………………………..………………….7 Weapons Caches Found and Cleared in Iraq, January 2004-Present……………………………………………NEW..………………………………….....7 Multiple Fatality Bombings in Iraq………………………………………………..………..…………………………………..……………..……..…………..8 Killed and Wounded in Multiple Fatality Bombings………………………………………………………………………………..…………………………...8 Multiple Fatality Bombings by Type Since January 2007…………………………………………………….…………………………………………………9 Detailed Breakdown of Deaths Associated with Multiple Fatality Bombings in Iraq……………………….…………………………………………..…....9 Number of Multiple Fatality Bombings Targeting Civilians by Sectarian Group and Month………………………………………………………………10 Number of Newly Displaced People Per Month in Iraq, Externally and Abroad…………………………………………..………………………………...10 -

High Casualty Terrorist Bombings (HCTB) Page 1 March 11, 1998 - March 10, 2020
High Casualty Terrorist Bombings (HCTB) Page 1 March 11, 1998 - March 10, 2020 LOC DEATH NINCD MONTH DAY YEAR LOCATION COL 25 3 5 13 1990 Cali, Niza, Quirigua USR 20 1 8 10 1990 Gyandzha, Azerbaijan COL 22 1 2 16 1991 Medellin TUR 36 1 4 9 1991 Istanbul IND 41 2 10 18 1991 Rudrapur ARG 28 1 3 17 1992 Buenos Aires PER 20 1 7 16 1992 Lima PAK 20 1 1 11 1993 Kotri PAK 23 2 1 24 1993 Hyderabad COL 20 1 1 30 1993 Bogota IND 317 13 3 12 1993 Bombay (Mumbai) IND 86 1 3 16 1993 Calcutta COL 15 1 4 15 1993 Bogota IRN 25 1 6 20 1994 Meshed ARG 96 1 7 18 1994 Buenos Aires PAN 21 1 7 19 1994 Colon ISR 23 1 10 19 1994 Tel Aviv ISR 22 2 1 22 1995 Netanya ALG 42 1 1 30 1995 Algiers IRQ 54 1 2 27 1995 Zakho USA 168 1 4 19 1995 Oklahoma City COL 29 1 6 11 1995 Medellin IND 17 1 7 20 1995 Jammu IND 16 1 8 31 1995 Chandigarh SRI 15 2 11 11 1995 Colombo PAK 17 1 11 19 1995 Islamabad IRQ 33 1 11 31 1995 Salahuddin PAK 45 1 12 21 1995 Peshawar SRI 96 1 1 31 1996 Colombo ISR 23 1 2 25 1996 Jerusalem ISR 19 1 3 3 1996 Jerusalem ISR 15 1 3 4 1996 Tel Aviv IND 25 1 3 21 1996 Delhi PAK 60 1 4 28 1996 Bhai Pheri SAU 19 1 6 25 1996 Dhahran SRI 64 1 7 24 1996 Dehiwala TUR 17 1 11 17 1996 Istanbul PER 17 1 12 17 1996 Lima IND 33 1 12 30 1996 Brahmaputra Mail (Assam) CAM 19 4 3 30 1997 Phnom Penh ALG 22 1 4 25 1997 Train near Algiers ALG 15 1 5 3 1997 Sidi Bouhanifa IND 33 1 7 8 1997 Bhatinda ISR 16 2 7 30 1997 Jerusalem SRI 18 1 10 15 1997 Colombo EGY 70 1 11 17 1997 Luxor ALG 103 1 1 11 1998 Sidi Hamed CHN 50 1 2 14 1998 Wuhan IND 46 13 2 14 1998 Coimbatore ALG 18 -

Reimagining the Character of Urban Operations for the U.S. Army: How the Past Can Inform the Present and Future
C O R P O R A T I O N Reimagining the Character of Urban Operations for the U.S. Army How the Past Can Inform the Present and Future Gian Gentile, David E. Johnson, Lisa Saum-Manning, Raphael S. Cohen, Shara Williams, Carrie Lee, Michael Shurkin, Brenna Allen, Sarah Soliman, James L. Doty III For more information on this publication, visit www.rand.org/t/RR1602 Library of Congress Cataloging-in-Publication Data is available for this publication. ISBN: 978-0-8330-9607-4 Published by the RAND Corporation, Santa Monica, Calif. © Copyright 2017 RAND Corporation R® is a registered trademark. Limited Print and Electronic Distribution Rights This document and trademark(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is required from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions. The RAND Corporation is a research organization that develops solutions to public policy challenges to help make communities throughout the world safer and more secure, healthier and more prosperous. RAND is nonprofit, nonpartisan, and committed to the public interest. RAND’s publications do not necessarily reflect the opinions of its research clients and sponsors. Support RAND Make a tax-deductible charitable contribution at www.rand.org/giving/contribute www.rand.org Preface The history of human conflict suggests that the U.S. -

From Dictatorship to Democracy: Iraq Under Erasure Abeer Shaheen
From Dictatorship to Democracy: Iraq under Erasure Abeer Shaheen Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2015 ©2015 Abeer Shaheen All rights reserved ABSTRACT From Dictatorship to Democracy: Iraq under Erasure Abeer Shaheen This dissertation examines the American project in Iraq between 1991 and 2006. It studies the project’s conceptual arc, shifting ontology, discourses, institutions, practices, and technologies in their interrelatedness to constitute a new Iraq. It is an ethnography of a thixotropic regime of law and order in translation; a circuit through various landscapes and temporalities to narrate the 1991 war, the institutionalization of sanctions and inspection regimes, material transformations within the American military, the 2003 war and finally the nation- building processes as a continuous and unitary project. The dissertation makes three central arguments: First, the 2003 war on Iraq was imagined through intricate and fluid spaces and temporalities. Transforming Iraq into a democratic regime has served as a catalyst for transforming the American military organization and the international legal system. Second, this project has reordered the spatialized time of Iraq by the imposition of models in translation, reconfigured and reimagined through a realm of violence. These models have created in Iraq a regime of differential mobility, which was enabled through an ensemble of experts, new institutions and calculative technologies. Third, this ensemble took Iraq as its object of knowledge and change rendering Iraq and Iraqis into a set of abstractions within the three spaces under examination: the space of American military institutions; the space of international legality within the United Nations; and, lastly, the material space of Baghdad. -

Iraq- Baghdad Governorate, Resafa District (
( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( ( Iraq- Baghdad Governorate, Resafa District ( ( Tal Skhairy IQ-P08720 Turkey ( ( Ba'quba District Mosul! ! ) Erbil ﺑﻌﻘوﺑﺔ Sabe'a Diyala Governorate Syria Iran ( Qusor - 368 IQ-D058 Baghdad ! دﯾﺎﻟﻰ IQ-P08359 ( Ramadi !\ IQ-G10 ( Jordan Najaf! ( Qaryat Qal`at `Abd Al-Nahriyah Amar Bin Basrah! al Jasadi IQ-P12554 Yasir Village ( IQ-P08346 ( IQ-P08934 Arab Yahudah ( ( ( SaudiI QA-Pra11b9i0a5 Kuwait Al Sha'ab ( Al Sha'ab - 375 B Mojam' Al Baladrooz District (Mahala 351) IQ-P08281 IQ-P08282 ( Zahra - 329 ( ( ( IQ-P08338 ﺑﻠدروز ) Al Sha'ab Al Hamediya Hay Al - 339 Ur - 327 ( ( Aqari IQ-P08280 IQ-P08367 - 575 Al Hamediya IQ-D057 Basateen - ( Al Sha'ab IQ-P09077 - 575 IQ-P08964 ( ( Mahalla 348 Al Basaten Al ( - 331 IQ-P09078 Ma'amil - IQ-P08303 sadren complex IQ-P08278 Mahalla 799 ( ( IQ-P08998 IQ-P08237 Hay Ur ( ( ( IQ-P08983 Basaten - 362 Hay Al Al Wafa IQ-P08304 Tujjar Al Sha'ab complex - 333 Sector - Qaryat Um IQ-P08325 Al Rihlat illegal Mujamma' Al Sadir 8 - Al Bisatein IQ-P08291 5 Ma'amel Al-Ubaid Basateen ( Hay Adan ( - 333 ( Sharekah - 345 collective sector 74 Thawra (9) IQ-P09019 IQ-P08715 IQ-P08302 IQ-P08862( IQ-P08963 ( IQ-P08279 IQ-P08341 Thawra1 District ( ( ( IQ-P08274 IQ-P09090 IQ-P09075 ( ( Nazl Salman Hay Al Tujjar( ( Hay Al Sihaa Al Sha'ab ( ( ( ( ( ( Sadir 8 - اﻟﺛورة Afandi IQ-P08968 ( IQ-P08323 caravans complex 1 IQ-P08344 ( IQ-P08283 Sector 80 Tunis - 326 ( ( Tunis - 330 Hay Al Aqari IQ-P09091 IQ-P08364 (Mahalla 337) ( IQ-D046 IQ-P08365 ( Mujamma' Al Sadir 7 - IQ-P08316 Al Biydha'a Sheblah -

IRAQ - Baghdad Governorate - Baghdad City Production Date : 19 December 2017 REFERENCE MAP As of December 2017
For Humanitarian Purposes Only IRAQ - Baghdad Governorate - Baghdad City Production date : 19 December 2017 REFERENCE MAP As of December 2017 Neighbourhood Shaab North District Border Rashdiya Shaab South Kadhimiyah Shaab East Adhamia Krai'at Tunis Thawra2 Park Thawra1 Ur Education Kadimiyah Maghreb Touristic Kadimiyah 1 Qahira Thawra Dabbash Ishbilya Services Al Husseinia Kadimiyah 2 Waziriya-Industry Shuala Adhamiyah Hay 14 Waziriya July Al Sadr Habbibiyah Obaydi Bab Mustansiriya Railway Hurriyah Utayfia Al-Muadham Primary Road Idrissi Medical Sheik Rusafa Ali Al-Saleh Secondary Road Ghazaliya, City Omar Nile East City Lake/River Camp Games Abu Habibiyah Ghraib Sheik Sinak Gaylani Fedhailia Scout Camp Iskan / Gazaliya Al-Adil Maaruf Shawaka Khulani Shaab Stadium Washash Old Al Orphalia Resafa Al-Bakreeia Scout Camp Muthanna IDP Camp Camp New Gazaliya Salhia Resafa Al Saadoom Bagdad Airport IDP Camp Ghazaliya, Kadhra Park Muthanna (Under Construction) Camp West Al Khadhra'a Baghdad Abu Nuwas Zayona Mansour Zoo Karkh Al Jamea'a University Ghadeer 14 Ramadhan Al Zawra Sinaa' Mashtal Park Al-Kindi New Baghdad Mansour Safarat Camp Qadissiya Karrada In Al Amriya Complex Sarah Al Yarmuk Karrada out Karkh Um Al Khanzeer Island Al Rasheed Jihad Baghdad Camp Camp location: CCCM and REACH, as of University December2017 Mada'in Administrative boundaries: OCHA, USDOS Al Atiba'a Zuwiya Settlements: OSM Dora Refinery Roads and railways: OCHA, OSM Bajas Baghdad University Water areas: OCHA, OSM International Satellite Imagery: Sentinal-2 from Airport -

Backgrounder Baghdad Neighborhood Project: Rusafa
Backgrounder Baghdad Neighborhood Project: Rusafa Marisa Cochrane, Researcher, Institute for the Study of War Overview The Rusafa security district provides an interesting look at a complex Baghdad neighborhood with strategic significance and changing demographics; it is an area in which U.S. and Iraqi forces have sought to revive and stabilize the political and economic life, while combating extreme violence caused by Jaysh al-Madhi (JAM) militias and al-Qaeda insurgents. Rusafa is a mix of large markets, government ministries, bus stations, educational institutions such as Mustansiriya University, hotels, hospitals, and the Rule of Law Green Zone. Yet, the district has also been plagued by sectarian violence and deadly car bombs, which often target Rusafa’s markets and bus stations. Rusafa has, since February, become a focal point for Coalition clearing and reconstruction efforts and this work has continued over the last eight months.i Currently, U.S. soldiers from the 1 st Battalion, 504 th Parachute Infantry Regiment and the 2 nd Combined Arms Battalion, 69 th Arms Regiment, both attached to the 2 nd Infantry Brigade Combat Team, 2 nd Infantry Division, are responsible for Rusafa. Situated on the eastern bank of the Tigris River in the heart of the city, Rusafa, together with areas of the Kadimiya district opposite the Tigris, comprise what is also known as Old Baghdad, the historic capital of the Abbasid Empire. Thus, the area along the river is composed of narrow and winding lanes, bustling intersections, and historic sites. The district of Adhamiyah neighbors Rusafa to the north, and the districts of Karadah and 9 Nissan border to the south and southeast.