ABSTRACT MORPHOLOGY and INTROGRESSIVE HYBRIDIZATION in NORTH AMERICAN DIPHASIASTRUM by Laura L. Klein Although Interspecific
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lycopodium (Spp) L. Ground Pine
Kasey Hartz Natural Area Reference Sheet Lycopodium (spp) L. Ground pine. Lycopodiaceae (Club-moss Family) Blooming season: Not a flowering plant. Spores produced in July-September. Plant: Tree like in form, 10-25 cm tall. Horizontal stem, not a true rhizome; grows at one end only, and the opposite end dies, resulting in the plant slowly moving forward year by year (Billington 1952). Leaves: Evergreen, scale like. Flower: None, spores are produced on strobilus cones. Fruit: None, but what is formed are strobilus cones which are sessile, having no stalks. Usually 1-3 cones, 1.5-5.5 cm long, maturing July-September. Spores are sulphur yellow with a high oil content. Can be confused with: Many species of Lycopodium can be confused and hybrids may also be found. Geographic range: Type specimen location: State: Throughout. Regional: Newfoundland - Alaska, south to North Carolina and Indiana. Habitat: Local: Riparian. Regional: Moist woods. Common local companions: Pine, ferns, maples, and beech Kasey Hartz Natural Area Reference Sheet Lycopodium obscurum L. 2 Ground Pine Usages: Human: Because of the spores have a high oil content and are quite flammable, they were used as flash powder for the first photographic cameras(Harris 2003); for fireworks (Billington 1952); and to imitate lightning flashes for theatrical performances (Millspaugh 1892, 1974). Their oil content led to them being used by pharmacists in boxes of pills to prevent them sticking together (possibly a different species of Lycopodium). Medicinal uses in the past included treatments for gout, menstrual disorders, nervous disorders, fevers, and as a styptic and an emetic. -

RI Equisetopsida and Lycopodiopsida.Indd
IIntroductionntroduction byby FFrancisrancis UnderwoodUnderwood Rhode Island Equisetopsida, Lycopodiopsida and Isoetopsida Special Th anks to the following for giving permission for the use their images. Robbin Moran New York Botanical Garden George Yatskievych and Ann Larson Missouri Botanical Garden Jan De Laet, plantsystematics.org Th is pdf is a companion publication to Rhode Island Equisetopsida, Lycopodiopsida & Isoetopsida at among-ri-wildfl owers.org Th e Elfi n Press 2016 Introduction Formerly known as fern allies, Horsetails, Club-mosses, Fir-mosses, Spike-mosses and Quillworts are plants that have an alternate generation life-cycle similar to ferns, having both sporophyte and gametophyte stages. Equisetopsida Horsetails date from the Devonian period (416 to 359 million years ago) in earth’s history where they were trees up to 110 feet in height and helped to form the coal deposits of the Carboniferous period. Only one genus has survived to modern times (Equisetum). Horsetails Horsetails (Equisetum) have jointed stems with whorls of thin narrow leaves. In the sporophyte stage, they have a sterile and fertile form. Th ey produce only one type of spore. While the gametophytes produced from the spores appear to be plentiful, the successful reproduction of the sporophyte form is low with most Horsetails reproducing vegetatively. Lycopodiopsida Lycopodiopsida includes the clubmosses (Dendrolycopodium, Diphasiastrum, Lycopodiella, Lycopodium , Spinulum) and Fir-mosses (Huperzia) Clubmosses Clubmosses are evergreen plants that produce only microspores that develop into a gametophyte capable of producing both sperm and egg cells. Club-mosses can produce the spores either in leaf axils or at the top of their stems. Th e spore capsules form in a cone-like structures (strobili) at the top of the plants. -

Download Document
African countries and neighbouring islands covered by the Synopsis. S T R E L I T Z I A 23 Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands by J.P. Roux Pretoria 2009 S T R E L I T Z I A This series has replaced Memoirs of the Botanical Survey of South Africa and Annals of the Kirstenbosch Botanic Gardens which SANBI inherited from its predecessor organisations. The plant genus Strelitzia occurs naturally in the eastern parts of southern Africa. It comprises three arborescent species, known as wild bananas, and two acaulescent species, known as crane flowers or bird-of-paradise flowers. The logo of the South African National Biodiversity Institute is based on the striking inflorescence of Strelitzia reginae, a native of the Eastern Cape and KwaZulu-Natal that has become a garden favourite worldwide. It sym- bolises the commitment of the Institute to champion the exploration, conservation, sustain- able use, appreciation and enjoyment of South Africa’s exceptionally rich biodiversity for all people. J.P. Roux South African National Biodiversity Institute, Compton Herbarium, Cape Town SCIENTIFIC EDITOR: Gerrit Germishuizen TECHNICAL EDITOR: Emsie du Plessis DESIGN & LAYOUT: Elizma Fouché COVER DESIGN: Elizma Fouché, incorporating Blechnum palmiforme on Gough Island PHOTOGRAPHS J.P. Roux Citing this publication ROUX, J.P. 2009. Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands. Strelitzia 23. South African National Biodiversity Institute, Pretoria. ISBN: 978-1-919976-48-8 © Published by: South African National Biodiversity Institute. Obtainable from: SANBI Bookshop, Private Bag X101, Pretoria, 0001 South Africa. -

Effects of Lycopodium Clavatum and Equisetum Arvense Extracts from Western Romania
Romanian Biotechnological Letters Vol. , No. x, Copyright © 2016 University of Bucharest Printed in Romania. All rights reserved ORIGINAL PAPER Effects of Lycopodium clavatum and equisetum arvense extracts from western Romania Received for publication, July, 07, 2014 Accepted, October, 13, 2015 MARIA SUCIU1, FELIX AUREL MIC1, LUCIAN BARBU-TUDORAN2, VASILE MUNTEAN2, ALEXANDRA TEODORA GRUIA3,* 1University of Medicine and Pharmacy “Victor Babes”, Department of Functional Sciences, Timisoara, 2, Eftimie Murgu Sq., Timisoara, 300041, Timis County, Romania 2Babes-Bolyai University, Biology and Geology Department, 5-7 Clinicilor Str., Cluj-Napoca, 400084, Cluj County, Romania. 3Emergency Clinical County Hospital Timisoara, Regional Centre for Transplant Immunology Department, 10, Iosif Bulbuca Blvd., Timisoara, 300736, Timis County, Romania. *Address for correspondence to: [email protected], 10, Iosif Bulbuca Blvd., Timisoara, 300736, Timis County, Romania. Abbreviations: ALT–alanin transaminases, AST–aspartate transaminases, GC-MS–gas chromatograph coupled with mass spectrometry. Abstract Plants have always excited interest because of their active principles that could be a source of healing in various affections. The aim of this study was to demonstrate that the hepatoprotective and antimicrobial effects of Lycopodium clavatum and Equisetum arvense from the Western parts of Romania (Arad County) are not as pronounced as described in literature, against xenobiotic intoxication or microbial infection. To identify the plants active compounds, -

Notes on Some Species of Diphasiastrum
Preslia, Praha, 47: 232 - 240, 1975 Notes on some species of Diphasiastrum Poznamky k n~kterym druhum rodu Dipha11iaatrum Josef Holub HOLUB J. (1975): Notes on some species of Diphasiastrum. - Preslia, Praha, 47: 232- 240. Taxonomic and nomenclatural problems of some species of Diphasiastrum HOLUB are discussed. A special attention is pa.id to the interspecies D. / X / issleri and D. / x / zei leri. Original plants of D. / x / issleri correspond to the combination D. alpinum - D. complanatum. Plants corresponding to the combination D. alpinum - D. tristachyum have been collected in the ~umava Mts. Some taxa described from the subarctic regions of Europe and North America are shown to belong most probably to the neglected interspecies D. / x / zeileri. Botanical I nstitute, Czechoslovak Academy of Sciences, 25~ 43 Prithonice, Czecho.,lovakia. INTRODUCTION This is a second part of my study of the new genus Diphasiastrum (HOLUB 1975), which could not be published in this journal in its entirety. Notes on taxonomy and nomenclature are selected from materials gathered originally for my "Catalogue of Czechoslovak vascular plants". With regard to the character of that work the present observations summarize the results of my own studies and suggests problems to be studied in the future. OBSERVATIONS f!.iphasiastrum alpinum (L.) HOLUB Two varieties have been described in this species (both under the name Lycopodium alpi nttm L.): var. thellungii HERTER from Switzerland and var. planiramulosum TAKEDA from Japan. Both these taxa, especially the first one, require a taxonomic revision; the possibility cannot be exclu<led that they are conspecific with D. / x / issleri. -

Climate Change Vulnerability in Biodiversity Sector
Ministry of Envrionment and Physical Planning of the Republic of Macedonia United Nations Development Programme Project 00075206 “Third National Report to UNFCCC” Project report Climate change vulnerability in Biodiversity sector Melovski Ljupčo, Matevski Vlado, Hristovski Slavčo Institute of Biology, Faculty of Natural Sciences and Mathematics, Ss. Cyril and Methodius University, Skopje, Republic of Macedonia Skopje, 2013 2 Contents 1 National Circumstances related to climate change vulnerability in Biodiversity Sector ................ 5 1.1 Introduction – climate change and Biodiversity Sector ................................................................ 5 1.1.1 Climate change vulnerability in Biodiversity Sector........................................................ 5 1.1.2 Climate change adaptation in Biodiversity Sector .......................................................... 7 1.2 Overview of Biodiversity Sector .................................................................................................... 7 1.2.1 Characteristics ................................................................................................................. 7 1.2.2 Major stakeholders ....................................................................................................... 15 1.2.3 Sector documents ......................................................................................................... 17 1.2.4 Data availability ............................................................................................................ -

(Lycopodiaceae) in the State of Veracruz, Mexico
Mongabay.com Open Access Journal - Tropical Conservation Science Vol.8 (1): 114-137, 2015 Research article Distribution and conservation status of Phlegmariurus (Lycopodiaceae) in the state of Veracruz, Mexico Samaria Armenta-Montero1, César I. Carvajal-Hernández1, Edward A. Ellis1 and Thorsten Krömer1* 1Centro de Investigaciones Tropicales, Universidad Veracruzana, Casco de la Ex Hacienda Lucas Martín, Privada de Araucarias S/N. Col. Periodistas, C.P. 91019, Xalapa, Veracruz, Mexico *Corresponding author. Email: [email protected] Abstract The fern and lycophyte flora of Mexico contains 13 species in the genus Phlegmariurus (Lycopodiaceae; club moss family), of which nine are found in the state of Veracruz (P. cuernavacensis, P. dichotomus, P. linifolius, P. myrsinites, P. orizabae, P. pithyoides, P. pringlei, P. reflexus , P. taxifolius). They are located primarily in undisturbed areas of humid montane, pine-oak and tropical humid forests, which are all ecosystems threatened by deforestation and fragmentation. The objective of this study was to evaluate and understand the distribution and conservation status of species of this genus in the state of Veracruz, Mexico. Using Maxent, probability distributions were modeled based on 173 herbarium specimens (25% from recent collections by the authors and/or collaborators), considering factors such as climate, elevation and vegetation cover. Additionally, anthropogenic impacts on the original habitat of each species were analyzed in order to assign threatened categories based on IUCN classifications at regional levels. Results show that potential distributions are located in the montane regions of the central and southern parts of the state. All nine Phlegmariurus species in Veracruz were found to be in some category of risk, with P. -

Ecology and Distribution of Lycopodiaceae Mirbel in Malaysia
Blumea 54, 2009: 269–271 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE doi:10.3767/000651909X476265 Ecology and distribution of Lycopodiaceae Mirbel in Malaysia G. Rusea1, K. Claysius1, S. Runi1,2, U. Joanes2, K.M. Haja Maideen3, A. Latiff 3 Key words Abstract This paper is the first account to discuss the distribution, ecology and habitats of the Lycopodiaceae in Malaysia. Lycopodiaceae are widely distributed throughout Malaysia with respect to altitudes and environmental distribution conditions but most abundantly found in hill forest and lower montane forest, terrestrial as well as epiphytic, in ecology shaded or semi-shaded places with relatively high humidity. Pahang in Peninsular Malaysia and Sabah in Borneo habitats have the highest species diversity in terms of the number of species collected. Lycopodiaceae Published on 30 October 2009 INTRODUCTION RESULTS AND DISCUSSION The Lycopodiaceae s.l. are an ancient (Correll 1956) and In Malaysia, the family comprises 32 species including 11 va- probably monophyletic family without close living relatives rieties that are found in various altitudes and vegetation types, and have a virtually cosmopolitan distribution (Øllgaard 1992). sometimes in a restricted area (Table 1). The estimated number of species ranges from approximately Lycopodiella cernua has the widest distribution in Malaysia 300 to more than 400 around the world (Wikström 2001). It and is most common on acid soils and occurs along forest consists of three genera namely Huperzia, Lycopodium and fringes, along roadside, hillsides and mountain slopes fol- Lycopodiella. Worldwide the estimated number of species for lowed by Huperzia carinata and H. pinifolia, which occur on both Lycopodium and Lycopodiella is about 40 (Wikström & tree branches. -

Ecophysiology of Four Co-Occurring Lycophyte Species: an Investigation of Functional Convergence
Research Article Ecophysiology of four co-occurring lycophyte species: an investigation of functional convergence Jacqlynn Zier, Bryce Belanger, Genevieve Trahan and James E. Watkins* Department of Biology, Colgate University, Hamilton, NY 13346, USA Received: 22 June 2015; Accepted: 7 November 2015; Published: 24 November 2015 Associate Editor: Tim J. Brodribb Citation: Zier J, Belanger B, Trahan G, Watkins JE. 2015. Ecophysiology of four co-occurring lycophyte species: an investigation of functional convergence. AoB PLANTS 7: plv137; doi:10.1093/aobpla/plv137 Abstract. Lycophytes are the most early divergent extant lineage of vascular land plants. The group has a broad global distribution ranging from tundra to tropical forests and can make up an important component of temperate northeast US forests. We know very little about the in situ ecophysiology of this group and apparently no study has eval- uated if lycophytes conform to functional patterns expected by the leaf economics spectrum hypothesis. To determine factors influencing photosynthetic capacity (Amax), we analysed several physiological traits related to photosynthesis to include stomatal, nutrient, vascular traits, and patterns of biomass distribution in four coexisting temperate lycophyte species: Lycopodium clavatum, Spinulum annotinum, Diphasiastrum digitatum and Dendrolycopodium dendroi- deum. We found no difference in maximum photosynthetic rates across species, yet wide variation in other traits. We also found that Amax was not related to leaf nitrogen concentration and is more tied to stomatal conductance, suggestive of a fundamentally different sets of constraints on photosynthesis in these lycophyte taxa compared with ferns and seed plants. These findings complement the hydropassive model of stomatal control in lycophytes and may reflect canaliza- tion of function in this group. -

Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- BIBLIOGRAPHY
Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- BIBLIOGRAPHY BIBLIOGRAPHY Ackerfield, J., and J. Wen. 2002. A morphometric analysis of Hedera L. (the ivy genus, Araliaceae) and its taxonomic implications. Adansonia 24: 197-212. Adams, P. 1961. Observations on the Sagittaria subulata complex. Rhodora 63: 247-265. Adams, R.M. II, and W.J. Dress. 1982. Nodding Lilium species of eastern North America (Liliaceae). Baileya 21: 165-188. Adams, R.P. 1986. Geographic variation in Juniperus silicicola and J. virginiana of the Southeastern United States: multivariant analyses of morphology and terpenoids. Taxon 35: 31-75. ------. 1995. Revisionary study of Caribbean species of Juniperus (Cupressaceae). Phytologia 78: 134-150. ------, and T. Demeke. 1993. Systematic relationships in Juniperus based on random amplified polymorphic DNAs (RAPDs). Taxon 42: 553-571. Adams, W.P. 1957. A revision of the genus Ascyrum (Hypericaceae). Rhodora 59: 73-95. ------. 1962. Studies in the Guttiferae. I. A synopsis of Hypericum section Myriandra. Contr. Gray Herbarium Harv. 182: 1-51. ------, and N.K.B. Robson. 1961. A re-evaluation of the generic status of Ascyrum and Crookea (Guttiferae). Rhodora 63: 10-16. Adams, W.P. 1973. Clusiaceae of the southeastern United States. J. Elisha Mitchell Sci. Soc. 89: 62-71. Adler, L. 1999. Polygonum perfoliatum (mile-a-minute weed). Chinquapin 7: 4. Aedo, C., J.J. Aldasoro, and C. Navarro. 1998. Taxonomic revision of Geranium sections Batrachioidea and Divaricata (Geraniaceae). Ann. Missouri Bot. Gard. 85: 594-630. Affolter, J.M. 1985. A monograph of the genus Lilaeopsis (Umbelliferae). Systematic Bot. Monographs 6. Ahles, H.E., and A.E. -
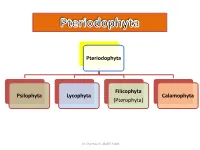
Pteriodophyta Psilophyta Lycophyta Filicophyta (Pterophyta) Calamophyta
Pteriodophyta Filicophyta Psilophyta Lycophyta Calamophyta (Pterophyta) Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora (Eligulatae) Order: Lycopodiales Family: Lycopodiaceae e.g. Lycopodium Subclass(2): Heterospora (Ligulatae) Order: Selaginellales Family: Selaginellaceae e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Phylum: Lepidophyta • It is characterized by: 1. The plant is differentiated into stem, leaves and roots. 2. Leaves are microphyllous (small, one vein and with no leaf gap). 3. The stele is protostele, siphonostele or polystele. 4. Protoxylem is exarch (ranging from a complete external cylinder to a polyarch stele in which there are many protoxylem ribs). 5. Sporangia are solitary, carried on special leaves (sporophylls). Sporophylls are usually collected in strobili. Dr. Shaimaa N. Abd El-Fatah . Among this phylum there are two independent evolutionary lines Heterospora (Ligulatae) Homospora (Eligulatae) • Characterized by presence • Eligulate (ligule lacking). of ligule (small outgrowth on • Homosporous (one type of the upper surface of the spores). leaf). • Heterosporous character (2 types of spores: microspores-- small, give rise to male gametophyte. & megaspores– larger, give rise to female gametophyte.) Dr. Shaimaa N. Abd El-Fatah • The phylum includes one class: Lycopodinae. • The class is classified into 4 orders: • Plants belong to the first order are homosporae (eligulatae), while the remaining three are heterosporae (ligulatae). Dr. Shaimaa N. Abd El-Fatah Class(2): Lycophyta Subclass(1): Homospora Subclass(2): Heterospora (Eligulatae) (Ligulatae) Order: Lycopodiales Order: Selaginellales Family: Lycopodiaceae Family: Selaginellaceae e.g. Lycopodium e.g. Selaginella Dr. Shaimaa N. Abd El-Fatah Lycopodiales This order is characterized by: 1. Homosporous and eligulatae. 2. Herbaceous without secondary growth. -

Conservation Assessment for Groundcedar and Stiff Clubmoss In
United States Department of Agriculture Conservation Assessment Forest Service for Groundcedar and Stiff Rocky Mountain Region Clubmoss in the Black Black Hills National Forest Hills National Forest South Custer, South Dakota Dakota and Wyoming March 2003 J.Hope Hornbeck, Deanna J. Reyher, Carolyn Sieg and Reed W. Crook Species Assessment of Groundcedar and Stiff Clubmoss in the Black Hills National Forest, South Dakota and Wyoming J. Hope Hornbeck, Deanna J. Reyher, Carolyn Hull Sieg and Reed W. Crook J. Hope Hornbeck is a Botanist with the Black Hills National Forest in Custer, South Dakota. She completed a B.S. in Environmental Biology at The University of Montana and a M.S. in Plant Biology at the University of Minnesota. Deanna J. Reyher is an Ecologist/Soil Scientist with the Black Hills National Forest in Custer, South Dakota. She completed a B.S. degree in Agronomy from the University of Nebraska. Carolyn Hull Sieg is a Research Plant Ecologist with the Rocky Mountain Research Station in Flagstaff, Arizona. She completed a B.S. in Wildlife Biology and M.S. in Range Science from Colorado State University and a Ph.D. in Range and Wildlife Management at Texas Tech University. Reed W. Crook is a Botanist with the Black Hills National Forest in Custer, South Dakota. He completed a B.S. in Botany at Brigham Young University, and a M.S. in Plant Morphology and Ph.D. in Plant Systematics at the University of Georgia. EXECUTIVE SUMMARY Stiff clubmoss (Lycopodium annotinum L.) and groundcedar (Lycopodium complanatum L.; synonym = Diphasiastrum complanatum [L.] Holub.) (Lycopodiaceae) are circumboreal clubmoss species that are widely distributed in North American boreal habitats.