HII Hep a Prophylaxis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -
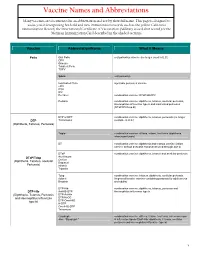
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

Adult Immunizations
Guidelines for Clinical Care Ambulatory Immunizations Guideline Team Adult Immunizations Team Leads Susan F Engert, MD, MPH Population: Adults, >18 years old Pediatrics and Communi- cable Diseases Objectives: Implement an evidence-based strategy for routine adult immunizations. Candia B Laughlin, RN, Key Points MS Routine immunizations for adults are: hepatitis A, hepatitis B, herpes zoster, human papilloma virus, Ambulatory Care Nursing Administration influenza, measles, mumps, rubella, meningococcal, pneumococcal, tetanus, diphtheria, pertussis and Team Members varicella. Below is a summary on priority populations, initial vaccination, and revaccination. Margie C Andreae, MD Use combination vaccines whenever possible to increase the coverage rates for vaccine-preventable Pediatrics and Communi- diseases: Tetanus-diphtheria (Td), Tetanus-diphtheria-acellular pertussis (Tdap), Measles-Mumps- cable Diseases Rubella (MMR), hepatitis A-hepatitis B (Twinrix®). Single antigen vaccines have no safety advantage. Mary K.Barry-Bodine, Live virus vaccines (Herpes Zoster, Measles-Mumps-Rubella, Varicella and Live Attenuated Influenza RN, BSN Vaccine) are contraindicated in persons who are pregnant or may become pregnant in the next four Nursing, Health Centers weeks, or who have immunocompromising conditions. If administering multiple live vaccines, give Susan G Blitz, MD, MPH simultaneously or separate them by 4 weeks. Tuberculosis (PPD) skin test should be administered General Medicine before or on the same day as a live virus vaccine or they need to be spaced 4-6 weeks apart. Katie Barwig, RN, MS This guideline follows recommendations of the federal Advisory Committee on Immunization Practices: Nursing Administration These vaccinations should be performed [strength of recommendation] for indicated populations at risk. Sherry L DeLoach, Evidence for each vaccine is based on randomized controlled trials [level of evidence] in general PharmD population and some subgroups, with findings extrapolated to some subgroups. -

(Nitags) and WHO Immunisation-Related Advisory Committees
Summary of recent issues considered by four national immunisation technical advisory groups (NITAGs) and WHO immunisation-related advisory committees Prepared by the National Centre for Immunisation Research & Surveillance (NCIRS) Period of review: 16/05/2019 to 13/09/2019 Contents 1 Advisory Committee on Immunization Practices (ACIP), USA .......................................................... 3 1.1 ACIP meeting: 26-27 June 2019 ....................................................................................................... 3 1.2 Newly published or updated recommendations ............................................................................... 20 1.2.1 Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunisation Practices.......................................................................................................................... 20 1.2.2 Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practice ..................................................................................................... 20 1.2.3 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2019–20 Influenza Season ............ 21 1.3 New or updated recommendations – not yet published ................................................................... 21 2 Immunisation Advisory Centre (IMAC), New Zealand .................................................................... -

Twinrix (Hepatitis a & B Combination)
TWINRIX (HEPATITIS A & B COMBINATION) Disease Risk & Transmission Hepatitis A (HAV) is a highly contagious liver disease with the common mode of transmission being person to person. Hepatitis A is found in the stool of infected persons and infection may be spread by contaminated water or food, by infected food handlers, after breakdown in sanitary conditions, or after floods or natural disasters, by ingestion of raw or uncooked shellfish from contaminated water, or by needle transmission (sharing needles, blood transfusions from infected persons), in daycare centers, during anal intercourse and during travel to countries in the world with poor hygienic conditions. A person who has Hepatitis A can easily spread on to others within the same household or dormitory. Symptoms include mild, flu-like symptoms, jaundice, decreased appetite, nausea and stomach pains, and diarrhea Hepatitis B (HBV) is an infection of the liver caused by the Hepatitis B virus. Hepatitis B is spread through contact with the blood of an infected person. Between 6-10 of every 100 young adults who have Hepatitis B become chronic carriers (have Hepatitis B in their blood for 6 or more months) and may be able to spread the infection to others for a long time. Hepatitis B is highly infective if there is contact with infected blood or serum through puncture wounds, splashes into the mucous membranes, contamination of open wounds, and injecting illegal drugs. It can be transmitted sexually, during birth and by sharing personal items such as razors and toothbrushes with an infected person. Symptoms include loss of appetite, tiredness, diarrhea & vomiting, pain in muscles and joints, dark urine & skin rashes. -

Hepatitis a Frequently Asked Questions
HEALTH NOTIFICATION FOR SOUTHERN NEVADA HEPATITIS A FREQUENTLY ASKED QUESTIONS WHAT ARE THE RECOMMENDATIONS is considered to be around 95 percent effective, that FOR HEPATITIS A VACCINE? protection will eventually begin to decrease and a Centers for Disease Control and Prevention (CDC) second shot boosts immunity for between 20 and 40 routinely recommends hepatitis A vaccination years, according to the CDC. for all children, for people who are at increased You can get a combination vaccine to protect you risk for infection, and for any people who want to against both hepatitis A and B. Check with your obtain immunity. health care provider. WHO SHOULD GET VACCINATED? CAN I GET MY HEPATITIS A SHOT During this current hepatitis A outbreak, the Health WHEN I GET OTHER IMMUNIZATIONS? District is working with community partners to You can receive the hepatitis A vaccine when you vaccinate people who are more likely to be infected receive other immunizations. The injection site will including people who use injection or non-injection be different. drugs (legal or illicit), people who are experiencing homelessness, and people who have recently been in IS THE HEPATITIS A VACCINE EFFECTIVE? jail or prison. Yes, the hepatitis A vaccine is highly effective in The CDC recommends hepatitis A vaccination for preventing hepatitis A virus infection. A second the following people: hepatitis A shot results in long-term protection. ¡ • All children at age of 1 year ¡ • People who are traveling to or working in countries IS THE HEPATITIS A VACCINE SAFE? where hepatitis A is common Yes, the hepatitis A vaccine is safe. -

Vaccine Adjuvants: from 1920 to 2015 and Beyond
Vaccines 2015, 3, 320-343; doi:10.3390/vaccines3020320 OPEN ACCESS vaccines ISSN 2076-393X www.mdpi.com/journal/vaccines Review Vaccine Adjuvants: from 1920 to 2015 and Beyond Alberta Di Pasquale 1,*, Scott Preiss 1, Fernanda Tavares Da Silva 1 and Nathalie Garçon 2 1 GSK Vaccines, Avenue Fleming, 1300 Wavre, Belgium; E-Mails: [email protected] (S.P.); [email protected] (F.T.D.S.) 2 Bioaster, 321 Avenue Jean Jaurès, 6700 Lyon, France; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +32-10-85-3573. Academic Editor: Diane M. Harper Received: 23 February 2015 / Accepted: 9 April 2015 / Published: 16 April 2015 Abstract: The concept of stimulating the body’s immune response is the basis underlying vaccination. Vaccines act by initiating the innate immune response and activating antigen presenting cells (APCs), thereby inducing a protective adaptive immune response to a pathogen antigen. Adjuvants are substances added to vaccines to enhance the immunogenicity of highly purified antigens that have insufficient immunostimulatory capabilities, and have been used in human vaccines for more than 90 years. While early adjuvants (aluminum, oil-in-water emulsions) were used empirically, rapidly increasing knowledge on how the immune system interacts with pathogens means that there is increased understanding of the role of adjuvants and how the formulation of modern vaccines can be better tailored towards the desired clinical benefit. Continuing safety evaluation of licensed vaccines containing adjuvants/adjuvant systems suggests that their individual benefit-risk profile remains favorable. -

Cervarix, Suspension for Intramuscular Injection
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ----------------------- WARNINGS AND PRECAUTIONS ----------------------- CERVARIX safely and effectively. See full prescribing information for • Because vaccinees may develop syncope, sometimes resulting in falling CERVARIX. with injury, observation for 15 minutes after administration is recommended. Syncope, sometimes associated with tonic-clonic CERVARIX [Human Papillomavirus Bivalent (Types 16 and 18) Vaccine, movements and other seizure-like activity, has been reported following Recombinant] vaccination with CERVARIX. When syncope is associated with tonic- Suspension for Intramuscular Injection clonic movements, the activity is usually transient and typically Initial U.S. Approval: 2009 responds to restoring cerebral perfusion by maintaining a supine or Trendelenburg position. (5.1) ----------------------------INDICATIONS AND USAGE ---------------------------- • The tip caps of the prefilled syringes contain natural rubber latex which CERVARIX is a vaccine indicated for the prevention of the following may cause allergic reactions. (5.2) diseases caused by oncogenic human papillomavirus (HPV) types 16 and 18: • cervical cancer, ------------------------------ ADVERSE REACTIONS ------------------------------ • cervical intraepithelial neoplasia (CIN) Grade 2 or worse and • Most common local adverse reactions in ≥20% of subjects were pain, adenocarcinoma in situ, and redness, and swelling at the injection site. (6.1) • cervical intraepithelial -

Global Advisory Committee on Vaccine Safety (GACVS)
Global Advisory Committee on Vaccine Safety (GACVS) Report on GACVS meeting 16-17 June 2010 Peter G. Smith 1 | GACVS June 2010 report– SAGE November 2010 Safety of pandemic influenza A (H1N1) vaccines >30 pandemic (H1N1) 2009 vaccines licensed worldwide. >350 million doses of pandemic influenza vaccines administered (as of June 2010). Passive surveillance data from North America, EU, Japan and China reviewed 2 | GACVS June 2010 report– SAGE November 2010 Safety of pandemic influenza A (H1N1) vaccines – GACVS advice To date, the safety data of pandemic (H1N1) 2009 vaccines are reassuring - no unexpected safety concerns have been identified. Risk of GBS, if any, has been no more than reported previously for some seasonal trivalent inactivated influenza vaccine - the committee is waiting results of ongoing studies. So far there are no safety signals among those vaccinated during pregnancy and their offspring. Prospectively agreed upon case definitions for adverse events (e.g., anaphylaxis, GBS, convulsion) facilitate global comparisons of the safety profiles of vaccines used in different countries. 3 | GACVS June 2010 report– SAGE November 2010 Febrile seizures following seasonal influenza vaccine in Australia Increased number of reports of fever and febrile convulsions in children <5 years of age following receipt of the 2010 seasonal inactivated influenza vaccine (Fluvax) made by CSL. Seasonal influenza vaccine suspended for children <5 years of age. GACVS advice: Not aware of reports of increased fever or febrile convulsions with other 2010 seasonal vaccines. Recommends review data on the use of 2010 seasonal vaccines elsewhere. 4 | GACVS June 2010 report– SAGE November 2010 Adventitious agent in rotavirus vaccines Review of Rotarix and RotaTeq following the 25 March 2010 review of Rotarix with respect to PCV (porcine circo-virus). -

General Vaccine Information
2015 Recommended Immunizations for Children from 7 Through 18 Years Old 7–10 YEARS 11-12 YEARS 13-18 YEARS Tdap 1 Tetanus, Diphtheria, Pertussis (Tdap) Vaccine Tdap Human Papillomavirus (HPV) Vaccine (3 Doses)2 HPV MCV4 Meningococcal Conjugate Vaccine (MCV4) Dose 13 MCV4 Dose 13 Booster at age 16 years Influenza (Yearly)4 Pneumococcal Vaccine5 Hepatitis A (HepA) Vaccine Series6 Hepatitis B (HepB) Vaccine Series Inactivated Polio Vaccine (IPV) Series Measles, Mumps, Rubella (MMR) Vaccine Series Varicella Vaccine Series These shaded boxes indicate when the vaccine is These shaded boxes indicate the These shaded boxes indicate the vaccine is recommended for children with certain health recommended for all children unless your doctor tells vaccine should be given if a child is conditions that put them at high risk for serious diseases. Note that healthy children can get the you that your child cannot safely receive the vaccine. catching-up on missed vaccines. HepA series6. See vaccine-specific recommendations at www.cdc.gov/vaccines/pubs/ACIP-list.htm. FOOTNOTES 1 Tdap vaccine is recommended at age 11 or 12 to protect against tetanus, diphtheria and pertussis. If your child has not received any or all of the DTaP vaccine series, or if you don’t know if your child has received these shots, your child needs a single dose of Tdap when they are 7 -10 years old. Talk to your child’s health care provider to find out if they need additional catch-up vaccines. 2 All 11 or 12 year olds – both girls and boys – should receive 3 doses of HPV vaccine to protect against HPV-related disease. -

Havrix Vaqta Twinrix Last Reviewed 02 April 2019 Last Revi
OREGON HEALTH AUTHORITY IMMUNIZATION PROGRAM HEPATITIS A VACCINES: Havrix® Vaqta® Twinrix® Last Reviewed 02 April 2019 Last Revised 22 April 2019 This order expires 31 July 2021 April 22, 2019 Additional language and tables regarding the recommendations of the Advisory Committee on Immunization Practices (ACIP) for the use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel.4 MMWR 2018;67:1216–1220. Postexposure Prophylaxis (PEP) against Hepatitis A Virus (HAV) Infection: 1. Initiate HAV PEP as soon as possible, within 2 weeks of exposure. 2. Give HepA vaccine to all persons aged >12 months regardless of risk group, with co-administration of immune globulin (IG) when indicated. 3. See table 4 in section IV D for risk assessment details. Preexposure Protection Against HAV Infection for Travelers ≥6 months of age see section IV B for details. 1. MMR and HepA vaccine may be given at different anatomical sites to infants 6– 11 months of age if indicated. 2. IG may be given simultaneously with HepA vaccine, at different anatomical sites, if indicated. 3. Do not use IG and MMR at the same visit. I. OREGON MODEL IMMUNIZATION PROTOCOL: 1. Check the ALERT Immunization Information System (IIS) to determine whether the patient needs this vaccine and any other vaccines. 2. Screen clients for contraindications and precautions. 3. Provide a current Vaccine Information Statement (VIS), and answer any questions. 4. Verify needle length for IM injection into the vastus lateralis or deltoid muscles. 5 5. Avoid injecting in the upper third of the deltoid muscle. Hepatitis A Vaccines Page | 2 of 20 6. -

ACIP Recommended Immunization Schedule for Children And
Morbidity and Mortality Weekly Report Weekly / Vol. 70 / No. 6 February 12, 2021 Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger — United States, 2021 A. Patricia Wodi, MD1; Kevin Ault, MD2; Paul Hunter, MD3; Veronica McNally, JD4; Peter G. Szilagyi, MD5; Henry Bernstein, DO6,7 At its October 2020 meeting, the Advisory Committee website (https://www.cdc.gov/vaccines/schedules). Health care on Immunization Practices* (ACIP) approved the 2021 providers are advised to use the tables and the notes together. Recommended Child and Adolescent Immunization This immunization schedule is recommended by ACIP Schedule for Ages 18 Years or Younger. After Emergency Use (https://www.cdc.gov/vaccines/acip) and approved by CDC Authorization of Pfizer-BioNTech COVID-19 vaccine by the (https://www.cdc.gov), the American Academy of Pediatrics Food and Drug Administration (FDA), ACIP issued an interim (https://www.aap.org), the American Academy of Family recommendation for use of Pfizer-BioNTech COVID-19 vac- cine in persons aged ≥16 years at its December 12, 2020, meeting (1). In addition, ACIP approved an amendment to INSIDE include COVID-19 vaccine recommendations in the child 193 Advisory Committee on Immunization Practices and adolescent immunization schedule. After Emergency Recommended Immunization Schedule for Adults Use Authorization of Moderna COVID-19 vaccine by Aged 19 Years or Older — United States, 2021 FDA, ACIP issued an interim recommendation for use of 197 Comorbidities Among Young Adults with Moderna COVID-19 vaccine in persons aged ≥18 years at its Congenital Heart Defects: Results from the December 19, 2020, emergency meeting (2).