Langhans-Type and Foreign-Body-Type Multinucleated Giant Cells in Cutaneous Lesions of Sarcoidosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thrombokinetics in Giant Cell Arteritis, with Special Reference to Corticosteroid Therapy
Ann Rheum Dis: first published as 10.1136/ard.38.3.244 on 1 June 1979. Downloaded from Annals of the Rheumatic Diseases, 38, 1979, 244-247 Thrombokinetics in giant cell arteritis, with special reference to corticosteroid therapy ANNA-LISA BERGSTROM, BENGT-AKE BENGTSSON, LARS-BERTIL OLSSON, BO-ERIC MALMVALL, AND JACK KUTTI From the Departments of Internal Medicine and Infectious Diseases, Eastern Hospital, University of Gothenburg, S-416 85 Gothenburg, Sweden SUMMARY Duplicate platelet survival studies were carried out on 8 patients with giant cell arteritis (GCA), once before the institution of any therapy, and the second time when they were in a com- pletely asymptomatic phase after having received corticosteroid treatment. The time interval be- tween the studies ranged between 5 and 14 months. In the first study the mean peripheral platelet count was 486 ±25 x 109/1 and in the second 326 ±25 x 109/1. The difference between the means was highly significant (P<0 001). The mean life-span of the platelets was normal in the duplicate experiments (6 * 7 ±0 * 3 and 7 *3 ±0 *4 days, respectively). Platelet production rate was significantly (P<0 001) raised in the first experiment but became normal in response to corticosteroid therapy. It is concluded that the thrombocytosis seen in GCA is reactive to the inflammation present in this disease, and it seems reasonable to assume that the reduction in the peripheral platelet count which occurs in response to corticosteroid therapy accurately reflects the clinical improvement of the patient. Thrombocytosis is frequently present in patients Table 1 Eight patients with GCA in whom duplicate platelet sutrvival studies were carried out with untreated giant cell arteritis (GCA) (Olhagen, http://ard.bmj.com/ 1963; Hamrin, 1972; Malmvall and Bengtsson, Patients Age Sex Time interval Maintenance. -

Pigmentation Associated Histopathological Variations in Sympathetic Ophthalmia
Br J Ophthalmol: first published as 10.1136/bjo.64.3.220 on 1 March 1980. Downloaded from British Jolarnal of Ophthalmology, 1980, 64, 220-222 Pigmentation associated histopathological variations in sympathetic ophthalmia GEORGE E. MARAK, JR., AND HIROSHI IKUI From the Department of Ophthalmology, Georgetown University, Washington, DC, USA, and the Department of Ophthalmology, Kyishia University, Fukiioka, Japani SUMMARY The severity of inflammation in sympathetic ophthalmia is related to the degree of pigmentation, and the granulomatous response seems to be related to pigmentation. Eosinophilia is also associated with pigmentation, but this association appears to be fortuitous and is a result of the association of eosinophilia with severity of the inflammation. Elsching presented his most confusing pearl to the reasonably be considered intermediate in pigmen- ophthalmological community when he proposed tation between North American black and white that sympathetic ophthalmia represented an autoim- populations. mune response to uveal pigment.12 Recent obser- The magnitude of choroidal thickening by the vations have raised considerable doubts about the inflammatory infiltrate, the intensity of tissue role of uveal pigment as the offending antigenin eosinophilia, and the proportion of the epithelioid sympathetic ophthalmia.34 The function of the uveal cell response in the choroid compared to the melanocytes in sympathetic ophthalmia is not well lymphocytic infiltration were rated by a single understood, but pigmentary disturbances occur -

A Review of the Evidence for and Against a Role for Mast Cells in Cutaneous Scarring and Fibrosis
International Journal of Molecular Sciences Review A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis Traci A. Wilgus 1,*, Sara Ud-Din 2 and Ardeshir Bayat 2,3 1 Department of Pathology, Ohio State University, Columbus, OH 43210, USA 2 Centre for Dermatology Research, NIHR Manchester Biomedical Research Centre, Plastic and Reconstructive Surgery Research, University of Manchester, Manchester M13 9PT, UK; [email protected] (S.U.-D.); [email protected] (A.B.) 3 MRC-SA Wound Healing Unit, Division of Dermatology, University of Cape Town, Observatory, Cape Town 7945, South Africa * Correspondence: [email protected]; Tel.: +1-614-366-8526 Received: 1 October 2020; Accepted: 12 December 2020; Published: 18 December 2020 Abstract: Scars are generated in mature skin as a result of the normal repair process, but the replacement of normal tissue with scar tissue can lead to biomechanical and functional deficiencies in the skin as well as psychological and social issues for patients that negatively affect quality of life. Abnormal scars, such as hypertrophic scars and keloids, and cutaneous fibrosis that develops in diseases such as systemic sclerosis and graft-versus-host disease can be even more challenging for patients. There is a large body of literature suggesting that inflammation promotes the deposition of scar tissue by fibroblasts. Mast cells represent one inflammatory cell type in particular that has been implicated in skin scarring and fibrosis. Most published studies in this area support a pro-fibrotic role for mast cells in the skin, as many mast cell-derived mediators stimulate fibroblast activity and studies generally indicate higher numbers of mast cells and/or mast cell activation in scars and fibrotic skin. -

Fine Structure and Histochemistry of Epithelioid Cells in Sarcoidosis
Thorax: first published as 10.1136/thx.29.1.115 on 1 January 1974. Downloaded from Thorax (1974), 29, 115. Fine structure and histochemistry of epithelioid cells in sarcoidosis E. M. VALERIE JAMES and W. JONES WILLIAMS Pathology Department, Welsh National School of Medicine, The Heath, Cardiff James, E. M. Valerie and Jones Williams, W. (1974). Thorax, 29, 115-120. Fine structure and histochemistry of epithelioid cells in sarcoidosis. In this fine structure study of epithelioid cells in the granulomata of sarcoidosis we confirm our previous findings that they appear to be synthesizing rather than phagocytosing cells. The numerous characteristic intracyto- plasmic vesicles, 0 5 ,u in size, are shown to contain mucoglycoprotein and only rarely acid phosphatase activity. Epithelioid cells show varying quantities of extravesicular acid phos- phatase activity which is sometimes on the outer surface of the protein-containing vesicles. The possible role of secretory products of epithelioid cells in the formation and persistence ofgranulomata is discussed. It is suggested that epithelioid cells may be forming lymphokines. We have previously demonstrated that the fine that of Ericsson and Trump (1965) using the lead structure of granulomata in sarcoidosis and tuber- nitrate Gomori technique. Substrate-free and sodium culosis (Jones Williams, Erasmus, James, and fluoride control blocks were also prepared. After being Davies, 1970), in the Kveim test lesion (Jones washed in acetate buffer solutions the tissues were post-fixed in Millonig's osmium tetroxide, dehydrated, Williams, 1972), and in chronic beryllium disease and embedded in Araldite. Sections were cut on an (Jones Williams, Fry, and James, 1972a) are LKB ultramicrotome and 0.5 ,u sections were stained http://thorax.bmj.com/ similar. -

Fundamentals of Dermatology Describing Rashes and Lesions
Dermatology for the Non-Dermatologist May 30 – June 3, 2018 - 1 - Fundamentals of Dermatology Describing Rashes and Lesions History remains ESSENTIAL to establish diagnosis – duration, treatments, prior history of skin conditions, drug use, systemic illness, etc., etc. Historical characteristics of lesions and rashes are also key elements of the description. Painful vs. painless? Pruritic? Burning sensation? Key descriptive elements – 1- definition and morphology of the lesion, 2- location and the extent of the disease. DEFINITIONS: Atrophy: Thinning of the epidermis and/or dermis causing a shiny appearance or fine wrinkling and/or depression of the skin (common causes: steroids, sudden weight gain, “stretch marks”) Bulla: Circumscribed superficial collection of fluid below or within the epidermis > 5mm (if <5mm vesicle), may be formed by the coalescence of vesicles (blister) Burrow: A linear, “threadlike” elevation of the skin, typically a few millimeters long. (scabies) Comedo: A plugged sebaceous follicle, such as closed (whitehead) & open comedones (blackhead) in acne Crust: Dried residue of serum, blood or pus (scab) Cyst: A circumscribed, usually slightly compressible, round, walled lesion, below the epidermis, may be filled with fluid or semi-solid material (sebaceous cyst, cystic acne) Dermatitis: nonspecific term for inflammation of the skin (many possible causes); may be a specific condition, e.g. atopic dermatitis Eczema: a generic term for acute or chronic inflammatory conditions of the skin. Typically appears erythematous, -

Review Article
J Clin Pathol: first published as 10.1136/jcp.36.7.723 on 1 July 1983. Downloaded from J Clin Pathol 1983;36:723-733 Review article Granulomatous inflammation- a review GERAINT T WILLIAMS, W JONES WILLIAMS From the Department ofPathology, Welsh National School ofMedicine, Cardiff SUMMARY The granulomatous inflammatory response is a special type of chronic inflammation characterised by often focal collections of macrophages, epithelioid cells and multinucleated giant cells. In this review the characteristics of these cells of the mononuclear phagocyte series are considered, with particular reference to the properties of epithelioid cells and the formation of multinucleated giant cells. The initiation and development of granulomatous inflammation is discussed, stressing the importance of persistence of the inciting agent and the complex role of the immune system, not only in the perpetuation of the granulomatous response but also in the development of necrosis and fibrosis. The granulomatous inflammatory response is Macrophages and the mononuclear phagocyte ubiquitous in pathology, being a manifestation of system many infective, toxic, allergic, autoimmune and neoplastic diseases and also conditions of unknown The name "mononuclear phagocyte system" was aetiology. Schistosomiasis, tuberculosis and leprosy, proposed in 1969 to describe the group of highly all infective granulomatous diseases, together affect phagocytic mononuclear cells and their precursors more than 200 million people worldwide, and which are widely distributed in the body, related by http://jcp.bmj.com/ granulomatous reactions to other irritants are a morphology and function, and which originate from regular occurrence in everyday clinical the bone marrow.' Macrophages, monocytes, pro- histopathology. A knowledge of the basic monocytes and their precursor monoblasts are pathophysiology of this distinctive tissue reaction is included, as are Kupffer cells and microglia. -

Advances in Seborrheic Keratosis
A CME/CE-Certified Supplement to Original Release Date: December 2018 Advances in Seborrheic Expiration Date: December 31, 2020 Estimated Time To Complete Activity: 1 hour Participants should read the activity information, Keratosis review the activity in its entirety, and complete the online post-test and evaluation. Upon completing this activity as designed and achieving a passing score on FACULTY the post-test, you will be directed to a Web page that will Joseph F. Fowler Jr, MD Michael S. Kaminer, MD allow you to receive your certificate of credit via e-mail Clinical Professor and Director Associate Clinical Professor of Dermatology or you may print it out at that time. Contact and Occupational Yale Medical School The online post-test and evaluation can be accessed Dermatology New Haven, Connecticut at http://tinyurl.com/SebK2018. University of Louisville School of Adjunct Assistant Professor of Medicine Medicine (Dermatology), Warren Alpert Medical School Inquiries about continuing medical education (CME) Louisville, Kentucky of Brown University accreditation may be directed to the University of Providence, Rhode Island Louisville Office of Continuing Medical Education & Professional Development (CME & PD) at cmepd@ louisville.edu or (502) 852-5329. Designation Statement eborrheic keratosis (SK) has been called keratinizing surface.12 They can develop virtually The University of Louisville School of Medicine the “Rodney Dangerfield of skin lesions”— anywhere except for the palms, soles, and mucous designates this Enduring material for a maximum of 9 1.0 AMA PRA Category 1 Credit(s)™. Physicians should it earns little respect (as a clinical concern) membranes, but are most commonly observed claim only the credit commensurate with the extent of Sbecause of its benignity, commonality, usual on the trunk and face.6,13 The tendency to develop their participation in the activity. -

Biomaterials and the Foreign Body Reaction: Surface Chemistry Dependent Macrophage Adhesion, Fusion, Apoptosis, and Cytokine Production
BIOMATERIALS AND THE FOREIGN BODY REACTION: SURFACE CHEMISTRY DEPENDENT MACROPHAGE ADHESION, FUSION, APOPTOSIS, AND CYTOKINE PRODUCTION by JACQUELINE ANN JONES Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy Dissertation Advisor: James Morley Anderson, M.D., Ph.D. Department of Biomedical Engineering CASE WESTERN RESERVE UNIVERSITY May, 2007 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the dissertation of ______________________________________________________ candidate for the Ph.D. degree *. (signed)_______________________________________________ (chair of the committee) ________________________________________________ ________________________________________________ ________________________________________________ ________________________________________________ ________________________________________________ (date) _______________________ *We also certify that written approval has been obtained for any proprietary material contained therein. Copyright © 2007 by Jacqueline Ann Jones All rights reserved iii Dedication This work is dedicated to… My ever loving and ever supportive parents: My mother, Iris Quiñones Jones, who gave me the freedom to be and to dream, inspires me with her passion and courage, and taught me the true meaning of friendship. My father, Glen Michael Jones, a natural-born teacher who taught me to be an inquisitive student of life, inspires me with his strength and perseverance, and gave me his kind, earnest heart that loves deeply and always -

Linear Scleroderma “En Coup De Sabre” Coexisting with Plaque-Morphea
661 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.5.661 on 1 May 2003. Downloaded from SHORT REPORT Linear scleroderma “en coup de sabre” coexisting with plaque-morphea: neuroradiological manifestation and response to corticosteroids I Unterberger, E Trinka, K Engelhardt, A Muigg, P Eller, M Wagner, N Sepp, G Bauer ............................................................................................................................. J Neurol Neurosurg Psychiatry 2003;74:661–664 progression of skin lesions or any neurological abnormalities A 24 year old woman in the 33rd week of pregnancy during follow up. developed progressive neurological complications with On admission the patient was in the 33rd week of right sided hemiparesis in association with the occurrence pregnancy. She was fully oriented, had normal speech, and of linear scleroderma “en coup de sabre” (LSCS) and pre- presented with a right sided hemiparesis (grade 3/5, MRC existing plaque-morphea, already being treated by scale) with a positive Babinski sign on the right. There was no balneophototherapy. Further progression of neurological history of head trauma. symptoms led to a caesarean section with the delivery of a Routine laboratory testing and comprehensive serological healthy child. Brain magnetic resonance imaging (MRI) screening including antineutrophilic cytoplasmic antibodies showed focal T2 signal increases in the left frontoparietal (ANCA), anticardiolipin antibodies, and antibodies against region directly adjacent to the area of LSCS. -
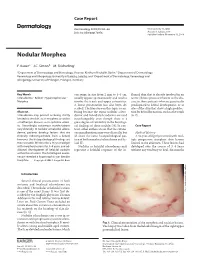
Nodular Morphea
Case Report Dermatology 2009;218:63–66 Received: July 13, 2008 DOI: 10.1159/000173976 Accepted: July 23, 2008 Published online: November 13, 2008 Nodular Morphea a b c F. Kauer J.C. Simon M. Sticherling a b Department of Dermatology and Venerology, Vivantes Klinikum Neukölln, Berlin , Department of Dermatology, c Venerology and Allergology, University of Leipzig, Leipzig , and Department of Dermatology, Venerology and Allergology, University of Erlangen, Erlangen , Germany Key Words can range in size from 2 mm to 4–5 cm, flamed skin that is already involved in an -Scleroderma ؒ Keloid ؒ Hypertrophic scar ؒ usually appear spontaneously and tend to active fibrotic process inherent to the dis Morphea involve the trunk and upper extremities. ease in those patients who are genetically A linear presentation has also been de- predisposed to keloid development, or at scribed. The literature on this topic is con- sites of the skin that show a high predilec- Abstract fusing because the terms ‘nodular sclero- tion for keloid formation, such as the trunk Scleroderma may present as being strictly derma’ and ‘keloidal scleroderma’ are used [6, 7] . limited to the skin, as in morphea, or within interchangeably even though there is a a multiorgan disease, as in systemic sclero- great degree of variability in the histologi- sis. Accordingly, cutaneous manifestations cal findings of these nodules [4] . In con- C a s e R e p o r t vary clinically. In nodular or keloidal sclero- trast, other authors stress that the cutane- derma, patients develop lesions that are ous manifestations may vary clinically, but Medical History clinically indistinguishable from a keloid; all share the same histopathological pat- A 16-year-old girl presented with mul- however, the histopathological findings are tern of both morphea/scleroderma and ke- tiple progressive morpheic skin lesions more variable. -

2016 Essentials of Dermatopathology Slide Library Handout Book
2016 Essentials of Dermatopathology Slide Library Handout Book April 8-10, 2016 JW Marriott Houston Downtown Houston, TX USA CASE #01 -- SLIDE #01 Diagnosis: Nodular fasciitis Case Summary: 12 year old male with a rapidly growing temple mass. Present for 4 weeks. Nodular fasciitis is a self-limited pseudosarcomatous proliferation that may cause clinical alarm due to its rapid growth. It is most common in young adults but occurs across a wide age range. This lesion is typically 3-5 cm and composed of bland fibroblasts and myofibroblasts without significant cytologic atypia arranged in a loose storiform pattern with areas of extravasated red blood cells. Mitoses may be numerous, but atypical mitotic figures are absent. Nodular fasciitis is a benign process, and recurrence is very rare (1%). Recent work has shown that the MYH9-USP6 gene fusion is present in approximately 90% of cases, and molecular techniques to show USP6 gene rearrangement may be a helpful ancillary tool in difficult cases or on small biopsy samples. Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors, 5th edition. Mosby Elsevier. 2008. Erickson-Johnson MR, Chou MM, Evers BR, Roth CW, Seys AR, Jin L, Ye Y, Lau AW, Wang X, Oliveira AM. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011 Oct;91(10):1427-33. Amary MF, Ye H, Berisha F, Tirabosco R, Presneau N, Flanagan AM. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013 Jul;463(1):97-8. CONTRIBUTED BY KAREN FRITCHIE, MD 1 CASE #02 -- SLIDE #02 Diagnosis: Cellular fibrous histiocytoma Case Summary: 12 year old female with wrist mass. -

Perivascular Epithelioid Cell Tumor of Gastrointestinal Tract Case Report and Review of the Literature
Perivascular Epithelioid Cell Tumor of Gastrointestinal Tract Case Report and Review of the Literature Biyan Lu, MD, PhD, Chenliang Wang, MD, Junxiao Zhang, MD, Roland P. Kuiper, PhD, Minmin Song, MD, Xiaoli Zhang, MD, Shunxin Song, MD, Ad Geurts van Kessel, PhD, Aikichi Iwamoto, MD, PhD, Jianping Wang, MD, PhD, and Huanliang Liu, MD, PhD Perivascular epithelioid cell tumors of gastrointestinal tract (GI PECo- markers. Molecular pathological assays confirmed a PSF-TFE3 gene mas) are exceedingly rare, with only a limited number of published fusion in one of our cases. Furthermore, in this case microphthalmia- reports worldwide. Given the scarcity of GI PEComas and their associated transcription factor and its downstream genes were found to relatively short follow-up periods, our current knowledge of their exhibit elevated transcript levels. biologic behavior, molecular genetic alterations, diagnostic criteria, Knowledge about the molecular genetic alterations in GI PEComas and prognostic factors continues to be very limited. is still limited and warrants further study. We present 2 cases of GI PEComas, one of which showed an (Medicine 94(3):e393) aggressive histologic behavior that underwent multiple combined che- motherapies. We also review the available English-language medical Abbreviations: AML = angiomyolipoma, CCMMT = clear cell literature on GI PEComas-not otherwise specified (PEComas-NOS) and myomelanocytic tumor of the falciform ligament/ligamentum teres, discuss their clinicopathological and molecular genetic features. CCST = clear cell ‘‘sugar’’ tumor of the lung, CDK2 = cyclin- Pathologic analyses including histomorphologic, immunohisto- dependent kinase 2, C-MET = met proto-oncogene, CRP = C- chemical, and ultrastructural studies were performed to evaluate the reactive protein, CT = computed tomography, DCT = dopachrome clinicopathological features of GI PEComas, their diagnosis, and differ- tautomerase, GAPDH = glyceraldehyde-phosphate dehydrogenase, ential diagnosis.