Taxonomic and Functional Diversity and Composition of Bats in a Regenerating Neotropical Dry Forest
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acoustic Identification of Mormoopid Bats: a Survey During the Evening Exodus
Journal of Mammalogy, 87(2):324–330, 2006 ACOUSTIC IDENTIFICATION OF MORMOOPID BATS: A SURVEY DURING THE EVENING EXODUS SILVIO MACI´AS,* EMANUEL C. MORA, AND ADIANEZ GARCI´A Department of Human and Animal Biology, Faculty of Biology, Havana University, CP 10 400, Ciudad de La Habana, Cuba (SM, ECM, AG) Department of Basic Formation, Faculty of Psychology, Havana University, Calle San Rafael No. 1168 entre Mazo´n y Basarrate, Centro Habana, Ciudad de La Habana, Cuba (SM) Echolocation calls emitted by the 4 species of Cuban mormoopid bats were compared to determine vocal signatures that enable identification of each species in the field during their evening exodus. Echolocation calls produced by Mormoops blainvilli are downward frequency-modulated (FM) signals in the range of 68.4– 52.5 kHz. Echolocation calls emitted by Pteronotus macleayii and P. quadridens have a similar design consisting of a short constant-frequency (CF) segment followed by a downward FM segment. The CF segment was at 70.0 kHz in calls from P. macleayii, and at 83.3 kHz in calls from P. quadridens. Echolocation calls from P. parnellii consist of a long CF segment, which is preceded by a short initial upward sweep and followed by a downward FM terminal sweep. The CF value of the 2nd harmonic was a good parameter for species identification. The features of the echolocation calls of each of the species were used to identify them during the evening exodus from 2 Cuban caves. Key words: echolocation, evening exodus, identification, mormoopid bats Effective monitoring of echolocation calls is vital in many families, such as Mormoopidae, should make it possible to use studies of the ecology and conservation of bats (Fenton 1997). -

Noctilio Leporinus (Chiroptera, Noctilionidae) from South America
AMERICAN MUSEUM NOVITATES Number 3798, 31 pp. April 4, 2014 Quaternary Bats from the Impossível-Ioiô Cave System (Chapada Diamantina, Brazil): Humeral Remains and the First Fossil Record of Noctilio leporinus (Chiroptera, Noctilionidae) from South America LEANDRO O. SALLES,1, 2 JOAQUÍN ARROYO-CABRALES,3 ANNE CARULINY DO MONTE LIMA,1 WAGNER LANZELOTTI,1 FERNANDO A. PERINI,4 PAÚL M. VELAZCO,2 AND NANCY B. SIMMONS2 ABSTRACT The partially submerged Impossível-Ioiô cave system located in the karst region of Cha- pada Diamantina in Bahia (Brazil) has recently been the target of extensive paleontological studies. Here we provide the first report of fossil bats from this cave system, in which we rec- ognize six species based on humeral remains: Furipterus horrens, Chrotopterus auritus, Mor- moops cf. megalophylla, Pteronotus gymnonotus, Pteronotus parnellii, and Noctilio leporinus. Morphology of the humerus of these taxa is described in a comparative framework to docu- ment taxonomic assessments and provide a basis for future studies of fossil bat faunas. The relevance of the new records reported here is evaluated at a broader continental scale, as well as in contrast with the recent bat fauna of the region. The record of Noctilio leporinus stands as the first fossil occurrence of this species on the South American continent. 1 Mastozoologia, Departamento de Vertebrados, Museu Nacional/UFRJ, Quinta da Boa Vista, s/n, Rio de Janeiro 20940-040, Brazil. 2 Division of Vertebrate Zoology (Mammalogy), American Museum of Natural History. 3 Laboratorio de Arqueozoología, Instituto Nacional de Antropología e Historia, Moneda # 16, Col. Centro México, D.F., México. 4 Departamento de Zoologia, Universidade Federal de Minas Gerais, Avenida Antônio Carlos 6627, Belo Horizonte 31270-901, Brazil. -

Cranial Morphology and Bite Force in Bats
CRANIOMANDIBULAR STRUCTURE AND FUNCTION IN MORMOOPID BATS A Thesis Presented to the faculty of the Department of Biological Sciences California State University, Sacramento Submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in Biological Sciences by Jeffrey B. Changaris FALL 2017 © 2017 Jeffrey B. Changaris ALL RIGHTS RESERVED ii CRANIOMANDIBULAR STRUCTURE AND FUNCTION IN MORMOOPID BATS A Thesis by Jeffrey B. Changaris Approved by: ____________________________________, Committee Chair Dr. Ronald M. Coleman ____________________________________, Second Reader Dr. Winston C. Lancaster ____________________________________, Third Reader Dr. Joseph Bahlman ___________________________ Date iii Student: Jeffrey B. Changaris I certify that this student has met the requirements for format contained in the University format manual, and that this thesis is suitable for shelving in the Library and credit is to be awarded for the thesis. ____________________________, Graduate Coordinator _________________ Dr. James W. Baxter Date Department of Biological Sciences iv Abstract of CRANIOMANDIBULAR STRUCTURE AND FUNCTION IN MORMOOPID BATS by Jeffrey B. Changaris Neotropical Ghost-Faced bats of the genus Mormoops (Order Chiroptera, Family Mormoopidae) have a radically upturned rostrum, or snout, while the other mormoopid genus, Pteronotus, has only a slight upturning of the rostrum. This type of difference in morphology between closely related taxa is likely to be the result of some sort of specialization. Observation of Mormoops blainvillei, the Antillean Ghost-Faced bat, reveals that they can open their mouths very wide relative to the size of their heads. Mormoopid bats are insectivorous with Mormoops blainvillei having a prey preference of large moths, but related species, such as Pteronotus quadridens, the Sooty Mustached bat, have a more varied diet with a large component of smaller hard-bodied beetles. -

Quaternary Bat Diversity in the Dominican Republic
AMERICAN MUSEUM NOVITATES Number 3779, 20 pp. June 21, 2013 Quaternary Bat Diversity in the Dominican Republic PAÚL M. VELAZCO,1 HANNAH O’NEILL,2 GREGG F. GUNNELL,3 SIOBHÁN B. COOKE,4 RENATO RIMOLI,5 ALFRED L. ROSENBErgER,1, 6 AND NANCY B. SIMMONS1 ABSTRACT The fossil record of bats is extensive in the Caribbean, but few fossils have previously been reported from the Dominican Republic. In this paper, we describe new collections of fossil bats from two flooded caves in the Dominican Republic, and summarize previous finds from the Island of Hispaniola. The new collections were evaluated in the context of extant and fossil faunas of the Greater Antilles to provide information on the evolution of the bat community of Hispaniola. Eleven species were identified within the new collections, including five mormoopids (Mormoops blainvillei, †Mormoops magna, Pteronotus macleayii, P. parnellii, and P. quadridens), five phyllostomids (Brachy- phylla nana, Monophyllus redmani, Phyllonycteris poeyi, Erophylla bombifrons, and Phyllops falcatus), and one natalid (Chilonatalus micropus). All of these species today inhabitant Hispaniola with the exception of †Mormoops magna, an extinct species previously known only from the Quaternary of Cuba, and Pteronotus macleayii, which is currently known only from extant populations in Cuba and Jamaica, although Quaternary fossils have also been recovered in the Bahamas. Differences between the fossil faunas and those known from the island today suggest that dispersal and extirpa- tion events, perhaps linked to climate change or stochastic events such as hurricanes, may have played roles in structuring the modern fauna of Hispaniola. 1 Division of Vertebrate Zoology (Mammalogy), American Museum of Natural History. -

WOOLLY FALSE VAMPIRE Chrotopterus Auritus (W.Peters, 1856)
Smith P - Chrotopterus auritus - FAUNA Paraguay Handbook of the Mammals of Paraguay Number 24 2008 WOOLLY FALSE VAMPIRE Chrotopterus auritus (W.Peters, 1856) FIGURE 1 - Head detail (© Merlin D. Tuttle, Bat Conservation International, www.batcon.org). TAXONOMY: Class Mammalia; Subclass Theria; Infraclass Metatheria; Order Chiroptera; Suborder Microchiroptera; Superfamily Noctilionoidea; Family Phyllostomidae; Subfamily Phyllostominae, Tribe Vampyrini (López-Gonzalez 2005, Myers et al 2006, Hoofer et al 2008). This species is the sole representative of the genus Chrotopterus, Peters 1865. The origin of the name Chrotopterus is Greek meaning "skin colour wing" presumably in reference to the wing membranes (Palmer 1904). The species name auritus is Latin meaning "long-eared". (Braun & Mares 1995). Czaplewski & Cartelle (1998) describe Quaternary fossils of this species from Minas Gerais, São Paulo and Bahía, Brazil. Carter & Dolan (1978) disputed the designation of Mexico as the type locality and claimed that the type specimen actually came from Santa Catarina, Brazil. However Peters (1856) clearly states that the specimen is from Mexico and Medellín (1989) claims that the type specimen is ZMB 10058 in the Zoologisches Museum der Humboldt Universität zu Berlin, which consists of a clean skull and skeleton with body parts in alcohol (Gardner 2007). Traditionally three subspecies have been recognised, that present in Paraguay is C.a.australis Thomas 1905 (Type Locality Concepción, Paraguay). Supposedly this subspecies is distinguished by its extensive woolly grey pelage which covers the wing and tail membranes reaching the elbows and knees, a Smith P 2008 - WOOLLY FALSE VAMPIRE Chrotopterus auritus - Mammals of Paraguay Nº 24 Page 1 Smith P - Chrotopterus auritus - FAUNA Paraguay Handbook of the Mammals of Paraguay Number 24 2008 small white spot on the wing tips and a woolly patch on the metacarpal of the thumb. -

Echolocation Calls and Wing Morphology of Bats from the West Indies
Acta Chiropterologica, 6(1): 75–90, 2004 PL ISSN 1508–1109 © Museum and Institute of Zoology PAS Echolocation calls and wing morphology of bats from the West Indies NANCY VAUGHAN JENNINGS1, STUART PARSONS2, KATE E. BARLOW3, and MICHAEL R. GANNON4 1School of Biological Sciences, University of Bristol, Woodland Road, Bristol BS8 1UG, United Kingdom E-mail: [email protected] 2School of Biological Sciences, University of Auckland, Private Bag 92019, Auckland, New Zealand 32 The Paddock, Seton Mains, Longniddry, East Lothian, EH32 0PG, United Kingdom Previous address: School of Biological Sciences, University of Bristol, Woodland Road, Bristol BS8 1UG, United Kingdom 4Department of Biology, The Pennsylvania State University, Altoona College, 3000 Ivyside Park Altoona, PA 16601-3760, USA Echolocation calls of 119 bats belonging to 12 species in three families from Antillean islands of Puerto Rico, Dominica, and St. Vincent were recorded by using time-expansion methods. Spectrograms of calls and descriptive statistics of five temporal and frequency variables measured from calls are presented. The echolocation calls of many of these species, particularly those in the family Phyllostomidae, have not been described previously. The wing morphology of each taxon is described and related to the structure of its echolocation calls and its foraging ecology. Of slow aerial-hawking insectivores, the Mormoopidae and Natalidae Mormoops blainvillii, Pteronotus davyi davyi, P. quadridens fuliginosus, and Natalus stramineus stramineus can forage with great manoeuvrability in background-cluttered space (close to vegetation), and are able to hover. Pteronotus parnellii portoricensis is able to fly and echolocate in highly-cluttered space (dense vegetation). Among frugivores, nectarivores and omnivores in the family Phyllostomidae, Brachyphylla cavernarum intermedia is adapted to foraging in the edges of vegetation in background-cluttered space, while Erophylla bombifrons bombifrons, Glossophaga longirostris rostrata, Artibeus jamaicensis jamaicensis, A. -

Broad Host Range of SARS-Cov-2 Predicted by Comparative and Structural Analysis of ACE2 in Vertebrates
Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates Joana Damasa,1, Graham M. Hughesb,1, Kathleen C. Keoughc,d,1, Corrie A. Paintere,1, Nicole S. Perskyf,1, Marco Corboa, Michael Hillerg,h,i, Klaus-Peter Koepflij, Andreas R. Pfenningk, Huabin Zhaol,m, Diane P. Genereuxn, Ross Swoffordn, Katherine S. Pollardd,o,p, Oliver A. Ryderq,r, Martin T. Nweeias,t,u, Kerstin Lindblad-Tohn,v, Emma C. Teelingb, Elinor K. Karlssonn,w,x, and Harris A. Lewina,y,z,2 aThe Genome Center, University of California, Davis, CA 95616; bSchool of Biology and Environmental Science, University College Dublin, Belfield, Dublin 4, Ireland; cGraduate Program in Pharmaceutical Sciences and Pharmacogenomics, Quantitative Biosciences Consortium, University of California, San Francisco, CA 94117; dGladstone Institute of Data Science and Biotechnology, San Francisco, CA 94158; eCancer Program, Broad Institute of MIT and Harvard, Cambridge, MA 02142; fGenetic Perturbation Platform, Broad Institute of MIT and Harvard, Cambridge, MA 02142; gMax Planck Institute of Molecular Cell Biology and Genetics, 01307 Dresden, Germany; hMax Planck Institute for the Physics of Complex Systems, 01187 Dresden, Germany; iCenter for Systems Biology Dresden, 01307 Dresden, Germany; jCenter for Species Survival, Smithsonian Conservation Biology Institute, National Zoological Park, Front Royal, VA 22630; kDepartment of Computational Biology, School of Computer Science, Carnegie Mellon University, Pittsburgh, PA 15213; lDepartment of Ecology, -

Caribbean Naturalist No
Caribbean Naturalist No. 35 2016 Conservation Value of Remnant Habitat for Neotropical Bats on Islands Armando Rodríguez-Durán and Waldemar Feliciano-Robles The Caribbean Naturalist . ♦ A peer-reviewed and edited interdisciplinary natural history science journal with a re- gional focus on the Caribbean ( ISSN 2326-7119 [online]). ♦ Featuring research articles, notes, and research summaries on terrestrial, fresh-water, and marine organisms, and their habitats. The journal's versatility also extends to pub- lishing symposium proceedings or other collections of related papers as special issues. ♦ Focusing on field ecology, biology, behavior, biogeography, taxonomy, evolution, anatomy, physiology, geology, and related fields. Manuscripts on genetics, molecular biology, anthropology, etc., are welcome, especially if they provide natural history in- sights that are of interest to field scientists. ♦ Offers authors the option of publishing large maps, data tables, audio and video clips, and even powerpoint presentations as online supplemental files. ♦ Proposals for Special Issues are welcome. ♦ Arrangements for indexing through a wide range of services, including Web of Knowledge (includes Web of Science, Current Contents Connect, Biological Ab- stracts, BIOSIS Citation Index, BIOSIS Previews, CAB Abstracts), PROQUEST, SCOPUS, BIOBASE, EMBiology, Current Awareness in Biological Sciences (CABS), EBSCOHost, VINITI (All-Russian Institute of Scientific and Technical Information), FFAB (Fish, Fisheries, and Aquatic Biodiversity Worldwide), WOW (Waters and Oceans Worldwide), and Zoological Record, are being pursued. ♦ The journal staff is pleased to discuss ideas for manuscripts and to assist during all stages of manuscript preparation. The journal has a mandatory page charge to help defray a portion of the costs of publishing the manuscript. Instructions for Authors are available online on the journal’s website (www.eaglehill.us/cana). -
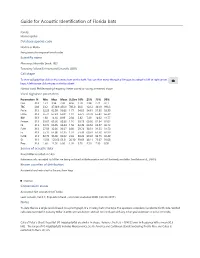
Mormoops Blainvillei Leach, 1821 Taxonomy Follows Simmons and Cirranello (2021) Call Shape
Guide for Acoustic Identification of Florida bats Family: Mormoopidae Database species code Morbla or Moba See glossary for explanation of codes Scientific name Mormoops blainvillei Leach, 1821 Taxonomy follows Simmons and Cirranello (2021) Call shape To view call graphics click on the camera icon on the right. You can then move through all images by using the left or right arrow keys. A left mouse click returns to the fact sheet. Narrow band, FM decreasing frequency. Never paired or having a reverse J shape Vocal signature parameters Parameters N Min Max Mean St.Dev 10% 25% 75% 90% Dur 313 1.51 3.94 2.36 0.56 1.70 1.94 2.71 3.17 TBC 238 33.2 4730.9 456.9 785.0 60.0 122.2 333.9 993.3 Fmin 313 52.29 62.99 56.03 1.77 54.05 54.61 57.35 58.39 Fmax 313 56.74 67.23 65.01 1.47 63.75 64.78 65.84 66.39 BW 313 1.38 14.10 8.99 2.30 5.82 7.49 10.62 11.77 Fmean 313 55.67 63.66 60.86 1.16 59.73 60.36 61.54 62.02 Fk 313 56.74 66.95 64.84 1.50 63.49 64.52 65.57 66.12 FcH1 313 27.59 32.00 30.67 0.80 29.74 30.31 31.25 31.50 Fc 313 55.17 64.00 61.35 1.59 59.48 60.61 62.50 62.99 FcH3 313 82.76 96.00 92.02 2.39 89.22 90.92 93.75 94.49 Sc 313 15.56 125.66 63.51 20.30 39.69 48.11 75.67 91.08 Pmc 313 1.60 14.50 6.00 1.95 3.70 4.50 7.30 8.50 Source of acoustic data Bruce Miller recorded in Cuba Reference calls recorded by Miller are being archived at BioAcoustica and will be freely available. -

Lessons from Bat Echolocationq
Animal Behaviour 85 (2013) 869e879 Contents lists available at SciVerse ScienceDirect Animal Behaviour journal homepage: www.elsevier.com/locate/anbehav Anniversary Essay Questions, ideas and tools: lessons from bat echolocationq M. Brock Fenton* Department of Biology, University of Western Ontario London, ON, Canada article info In their 1960 paper about bats using echolocation to find and track flying insects, Donald R. Griffin, e Article history: Fredric A. Webster and Charles R. Michael (Animal Behaviour, 8, 141 154) changed the face of research on Received 17 January 2013 this behaviour. They moved the field of echolocation from documenting that this animal or that one Initial acceptance 7 February 2013 could echolocate to demonstrating an adaptive value of echolocation. They used experiments with Final acceptance 12 February 2013 captive bats, fruit flies, mosquitoes and crane flies to illustrate how bats used a ‘feeding buzz’ as they Available online 27 March 2013 closed with their prey. The topic remains current today, and one of the first papers in Nature in 2013 MS. number: AAE-13-00056 (Jacobsen et al., 493, 93e96) presented more information about feeding buzzes building on the platform that Griffin et al. had established. In the intervening period, literally thousands of papers have been Keywords: published about echolocation, demonstrating how curious minds, technological advances and basic in- biosonar formation about natural history can result in diversification of a field of research. We have learned that communication bats can use echolocation to recognize water surfaces and to find insect prey on spider webs. The evolution feeding buzz continuum between orientation and social functions of echolocation means that this behaviour not only fl hearing in uences foraging and negotiating obstacle paths, but is also a cue that brings individuals together. -

3.9 Birds and Bats
Atlantic Fleet Training and Testing Draft EIS/OEIS June 2017 Draft Environmental Impact Statement/Overseas Environmental Impact Statement Atlantic Fleet Training and Testing TABLE OF CONTENTS 3.9 Birds and Bats .............................................................................................. 3.9-1 3.9.1 Introduction ........................................................................................................ 3.9-2 3.9.2 Affected Environment ......................................................................................... 3.9-3 3.9.2.1 General Background ........................................................................... 3.9-3 3.9.2.2 Endangered Species Act-Listed Species ............................................ 3.9-15 3.9.2.3 Species Not Listed Under the Endangered Species Act .................... 3.9-32 3.9.2.4 Migratory Birds ................................................................................. 3.9-46 3.9.3 Environmental Consequences .......................................................................... 3.9-49 3.9.3.1 Acoustic Stressors ............................................................................. 3.9-50 3.9.3.2 Explosive Stressors ............................................................................ 3.9-80 3.9.3.3 Energy Stressors ................................................................................ 3.9-87 3.9.3.4 Physical Disturbance and Strike Stressors ........................................ 3.9-95 3.9.3.5 Entanglement Stressors ................................................................. -

Monitoring Trends in Bat Populations of the United States and Territories: Problems and Prospects: U.S
MonitoringMonitoringMonitoring TTTrends in Bat Populations of the United SSthe tates andandtates TTTerritories:erritories:erritories: Problems and Prospects Information and Technology Report USGS/BRD/ITR–2003-0003 U.S. Department of the Interior U.S. Geological Survey To purchase this report, contact the National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161 (call toll free 1-800-553-6847), or the Defense Technical Information Center, 8725 Kingman Rd., Suite 0944, Fort Belvoir, VA 22060-6218. Cover photograph by Thomas J. O’Shea U.S. Geological Survey MonitoringMonitoringMonitoring TTTrends in Bat Populations of the United States andandtates TTTerritories:erritories:erritories: Problems and Prospects Information and Technology Report USGS/BRD/ITR–2003-0003 By T.J. O’Shea M.A. Bogan Editors U.S. Department of the Interior U.S. Geological Survey Suggested citation: O’Shea, T.J. and Bogan, M.A., eds., 2003, Monitoring trends in bat populations of the United States and territories: problems and prospects: U.S. Geological Survey, Biological Resources Discipline, Information and Technology Report, USGS/BRD/ITR--2003–0003, 274 p. ii ContentsContentsContents Page Introduction (T.J. O’Shea and M.A. Bogan) ............................................................................................ 1 Bats of the United States and Territories .......................................................................................................................2 Problems and Prospects for Monitoring Trends in Bat Populations