Comparative Time-Scale Gene Expression Analysis Highlights the Infection Processes of Two Amoebophrya Strains
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Molecular Data and the Evolutionary History of Dinoflagellates by Juan Fernando Saldarriaga Echavarria Diplom, Ruprecht-Karls-Un
Molecular data and the evolutionary history of dinoflagellates by Juan Fernando Saldarriaga Echavarria Diplom, Ruprecht-Karls-Universitat Heidelberg, 1993 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES Department of Botany We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA November 2003 © Juan Fernando Saldarriaga Echavarria, 2003 ABSTRACT New sequences of ribosomal and protein genes were combined with available morphological and paleontological data to produce a phylogenetic framework for dinoflagellates. The evolutionary history of some of the major morphological features of the group was then investigated in the light of that framework. Phylogenetic trees of dinoflagellates based on the small subunit ribosomal RNA gene (SSU) are generally poorly resolved but include many well- supported clades, and while combined analyses of SSU and LSU (large subunit ribosomal RNA) improve the support for several nodes, they are still generally unsatisfactory. Protein-gene based trees lack the degree of species representation necessary for meaningful in-group phylogenetic analyses, but do provide important insights to the phylogenetic position of dinoflagellates as a whole and on the identity of their close relatives. Molecular data agree with paleontology in suggesting an early evolutionary radiation of the group, but whereas paleontological data include only taxa with fossilizable cysts, the new data examined here establish that this radiation event included all dinokaryotic lineages, including athecate forms. Plastids were lost and replaced many times in dinoflagellates, a situation entirely unique for this group. Histones could well have been lost earlier in the lineage than previously assumed. -

The Planktonic Protist Interactome: Where Do We Stand After a Century of Research?
bioRxiv preprint doi: https://doi.org/10.1101/587352; this version posted May 2, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Bjorbækmo et al., 23.03.2019 – preprint copy - BioRxiv The planktonic protist interactome: where do we stand after a century of research? Marit F. Markussen Bjorbækmo1*, Andreas Evenstad1* and Line Lieblein Røsæg1*, Anders K. Krabberød1**, and Ramiro Logares2,1** 1 University of Oslo, Department of Biosciences, Section for Genetics and Evolutionary Biology (Evogene), Blindernv. 31, N- 0316 Oslo, Norway 2 Institut de Ciències del Mar (CSIC), Passeig Marítim de la Barceloneta, 37-49, ES-08003, Barcelona, Catalonia, Spain * The three authors contributed equally ** Corresponding authors: Ramiro Logares: Institute of Marine Sciences (ICM-CSIC), Passeig Marítim de la Barceloneta 37-49, 08003, Barcelona, Catalonia, Spain. Phone: 34-93-2309500; Fax: 34-93-2309555. [email protected] Anders K. Krabberød: University of Oslo, Department of Biosciences, Section for Genetics and Evolutionary Biology (Evogene), Blindernv. 31, N-0316 Oslo, Norway. Phone +47 22845986, Fax: +47 22854726. [email protected] Abstract Microbial interactions are crucial for Earth ecosystem function, yet our knowledge about them is limited and has so far mainly existed as scattered records. Here, we have surveyed the literature involving planktonic protist interactions and gathered the information in a manually curated Protist Interaction DAtabase (PIDA). In total, we have registered ~2,500 ecological interactions from ~500 publications, spanning the last 150 years. -

Metagenomic Characterization of Unicellular Eukaryotes in the Urban Thessaloniki Bay
Metagenomic characterization of unicellular eukaryotes in the urban Thessaloniki Bay George Tsipas SCHOOL OF ECONOMICS, BUSINESS ADMINISTRATION & LEGAL STUDIES A thesis submitted for the degree of Master of Science (MSc) in Bioeconomy Law, Regulation and Management May, 2019 Thessaloniki – Greece George Tsipas ’’Metagenomic characterization of unicellular eukaryotes in the urban Thessaloniki Bay’’ Student Name: George Tsipas SID: 268186037282 Supervisor: Prof. Dr. Savvas Genitsaris I hereby declare that the work submitted is mine and that where I have made use of another’s work, I have attributed the source(s) according to the Regulations set in the Student’s Handbook. May, 2019 Thessaloniki - Greece Page 2 of 63 George Tsipas ’’Metagenomic characterization of unicellular eukaryotes in the urban Thessaloniki Bay’’ 1. Abstract The present research investigates through metagenomics sequencing the unicellular protistan communities in Thermaikos Gulf. This research analyzes the diversity, composition and abundance in this marine environment. Water samples were collected monthly from April 2017 to February 2018 in the port of Thessaloniki (Harbor site, 40o 37’ 55 N, 22o 56’ 09 E). The extraction of DNA was completed as well as the sequencing was performed, before the downstream read processing and the taxonomic classification that was assigned using PR2 database. A total of 1248 Operational Taxonomic Units (OTUs) were detected but only 700 unicellular eukaryotes were analyzed, excluding unclassified OTUs, Metazoa and Streptophyta. In this research-based study the most abundant and diverse taxonomic groups were Dinoflagellata and Protalveolata. Specifically, the most abundant groups of all samples are Dinoflagellata with 190 OTUs (27.70%), Protalveolata with 139 OTUs (20.26%) Ochrophyta with 73 OTUs (10.64%), Cercozoa with 67 OTUs (9.77%) and Ciliophora with 64 OTUs (9.33%). -

Predatory Flagellates – the New Recently Discovered Deep Branches of the Eukaryotic Tree and Their Evolutionary and Ecological Significance
Protistology 14 (1), 15–22 (2020) Protistology Predatory flagellates – the new recently discovered deep branches of the eukaryotic tree and their evolutionary and ecological significance Denis V. Tikhonenkov Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, Borok, 152742, Russia | Submitted March 20, 2020 | Accepted April 6, 2020 | Summary Predatory protists are poorly studied, although they are often representing important deep-branching evolutionary lineages and new eukaryotic supergroups. This short review/opinion paper is inspired by the recent discoveries of various predatory flagellates, which form sister groups of the giant eukaryotic clusters on phylogenetic trees, and illustrate an ancestral state of one or another supergroup of eukaryotes. Here we discuss their evolutionary and ecological relevance and show that the study of such protists may be essential in addressing previously puzzling evolutionary problems, such as the origin of multicellular animals, the plastid spread trajectory, origins of photosynthesis and parasitism, evolution of mitochondrial genomes. Key words: evolution of eukaryotes, heterotrophic flagellates, mitochondrial genome, origin of animals, photosynthesis, predatory protists, tree of life Predatory flagellates and diversity of eu- of the hidden diversity of protists (Moon-van der karyotes Staay et al., 2000; López-García et al., 2001; Edg- comb et al., 2002; Massana et al., 2004; Richards The well-studied multicellular animals, plants and Bass, 2005; Tarbe et al., 2011; de Vargas et al., and fungi immediately come to mind when we hear 2015). In particular, several prevailing and very abun- the term “eukaryotes”. However, these groups of dant ribogroups such as MALV, MAST, MAOP, organisms represent a minority in the real diversity MAFO (marine alveolates, stramenopiles, opistho- of evolutionary lineages of eukaryotes. -
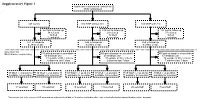
Supplementary Figure 1 Multicenter Randomised Control Trial 2746 Randomised
Supplementary Figure 1 Multicenter randomised control trial 2746 randomised 947 control 910 MNP without zinc 889 MNP with zinc 223 lost to follow up 219 lost to follow up 183 lost to follow up 34 refused 29 refused 37 refused 16 died 12 died 9 died 3 excluded 4 excluded 1 excluded 671 in follow-up 646 in follow-up 659 in follow-up at 24mo of age at 24mo of age at 24mo of age Selection for Microbiome sequencing 516 paired samples unavailable 469 paired samples unavailable 497 paired samples unavailable 69 antibiotic use 63 antibiotic use 67 antibiotic use 31 outside of WLZ criteria 37 outside of WLZ criteria 34 outside of WLZ criteria 6 diarrhea last 7 days 2 diarrhea last 7 days 7 diarrhea last 7 days 39 WLZ > -1 at 24 mo 10 WLZ < -2 at 24mo 58 WLZ > -1 at 24 mo 17 WLZ < -2 at 24mo 48 WLZ > -1 at 24 mo 8 WLZ < -2 at 24mo available for selection available for selection available for selection available for selection available for selection1 available for selection1 14 selected 10 selected 15 selected 14 selected 20 selected1 7 selected1 1 Two subjects (one in the reference WLZ group and one undernourished) had, at 12 months, no diarrhea within 1 day of stool collection but reported diarrhea within 7 days prior. Length, cm kg Weight, Supplementary Figure 2. Length (left) and weight (right) z-scores of children recruited into clinical trial NCT00705445 during the first 24 months of life. Median and quantile values are shown, with medians for participants profiled in current study indicated by red (undernourished) and black (reference WLZ) lines. -

Ellobiopsids of the Genus Thalassomyces Are Alveolates
J. Eukaryot. Microbiol., 51(2), 2004 pp. 246±252 q 2004 by the Society of Protozoologists Ellobiopsids of the Genus Thalassomyces are Alveolates JEFFREY D. SILBERMAN,a,b1 ALLEN G. COLLINS,c,2 LISA-ANN GERSHWIN,d,3 PATRICIA J. JOHNSONa and ANDREW J. ROGERe aDepartment of Microbiology, Immunology, and Molecular Genetics, University of California at Los Angeles, California, USA, and bInstitute of Geophysics and Planetary Physics, University of California at Los Angeles, California, USA, and cEcology, Behavior and Evolution Section, Division of Biology, University of California, La Jolla, California, USA, and dDepartment of Integrative Biology and Museum of Paleontology, University of California, Berkeley, California, USA, and eCanadian Institute for Advanced Research, Program in Evolutionary Biology, Genome Atlantic, Department of Biochemistry and Molecular Biology, Dalhousie University, Halifax, Nova Scotia, Canada ABSTRACT. Ellobiopsids are multinucleate protist parasites of aquatic crustaceans that possess a nutrient absorbing `root' inside the host and reproductive structures that protrude through the carapace. Ellobiopsids have variously been af®liated with fungi, `colorless algae', and dino¯agellates, although no morphological character has been identi®ed that de®nitively allies them with any particular eukaryotic lineage. The arrangement of the trailing and circumferential ¯agella of the rarely observed bi-¯agellated `zoospore' is reminiscent of dino¯agellate ¯agellation, but a well-organized `dinokaryotic nucleus' has never been observed. Using small subunit ribosomal RNA gene sequences from two species of Thalassomyces, phylogenetic analyses robustly place these ellobiopsid species among the alveolates (ciliates, apicomplexans, dino¯agellates and relatives) though without a clear af®liation to any established alveolate lineage. Our trees demonstrate that Thalassomyces fall within a dino¯agellate 1 apicomplexa 1 Perkinsidae 1 ``marine alveolate group 1'' clade, clustering most closely with dino¯agellates. -

Metabarcoding Analysis of Prey Composition of the Copepod Calanus Finmarchicus in Regions of the North Atlantic Ocean Heidi Yeh [email protected]
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by OpenCommons at University of Connecticut University of Connecticut OpenCommons@UConn Master's Theses University of Connecticut Graduate School 7-16-2018 Metabarcoding Analysis of Prey Composition of the Copepod Calanus finmarchicus in Regions of the North Atlantic Ocean Heidi Yeh [email protected] Recommended Citation Yeh, Heidi, "Metabarcoding Analysis of Prey Composition of the Copepod Calanus finmarchicus in Regions of the North Atlantic Ocean" (2018). Master's Theses. 1257. https://opencommons.uconn.edu/gs_theses/1257 This work is brought to you for free and open access by the University of Connecticut Graduate School at OpenCommons@UConn. It has been accepted for inclusion in Master's Theses by an authorized administrator of OpenCommons@UConn. For more information, please contact [email protected]. Metabarcoding Analysis of Prey Composition of the Copepod Calanus finmarchicus in Regions of the North Atlantic Ocean Heidi Yeh B.A., Barnard College, Columbia University, 2014 A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science At the University of Connecticut 2018 Copyright by Heidi Yeh 2018 ii APPROVAL PAGE Masters of Science Thesis Metabarcoding Analysis of Prey Composition of the Copepod Calanus finmarchicus in Regions of the North Atlantic Ocean Presented by Heidi Yeh, B.A. Major Advisor________________________________________________________________ Ann Bucklin Associate Advisor_______________________________________________________________ Senjie Lin Associate Advisor_______________________________________________________________ George McManus University of Connecticut 2018 iii ACKNOWLEDGEMENTS Many people have provided support and encouragement over the course of this research project. I would like to thank my advisor, Ann Bucklin. -

Genetic Diversity and Habitats of Two Enigmatic Marine Alveolate Lineages
AQUATIC MICROBIAL ECOLOGY Vol. 42: 277–291, 2006 Published March 29 Aquat Microb Ecol Genetic diversity and habitats of two enigmatic marine alveolate lineages Agnès Groisillier1, Ramon Massana2, Klaus Valentin3, Daniel Vaulot1, Laure Guillou1,* 1Station Biologique, UMR 7144, CNRS, and Université Pierre & Marie Curie, BP74, 29682 Roscoff Cedex, France 2Department de Biologia Marina i Oceanografia, Institut de Ciències del Mar, CMIMA, CSIC. Passeig Marítim de la Barceloneta 37-49, 08003 Barcelona, Spain 3Alfred Wegener Institute for Polar Research, Am Handelshafen 12, 27570 Bremerhaven, Germany ABSTRACT: Systematic sequencing of environmental SSU rDNA genes amplified from different marine ecosystems has uncovered novel eukaryotic lineages, in particular within the alveolate and stramenopile radiations. The ecological and geographic distribution of 2 novel alveolate lineages (called Group I and II in previous papers) is inferred from the analysis of 62 different environmental clone libraries from freshwater and marine habitats. These 2 lineages have been, up to now, retrieved exclusively from marine ecosystems, including oceanic and coastal waters, sediments, hydrothermal vents, and perma- nent anoxic deep waters and usually represent the most abundant eukaryotic lineages in environmen- tal genetic libraries. While Group I is only composed of environmental sequences (118 clones), Group II contains, besides environmental sequences (158 clones), sequences from described genera (8) (Hema- todinium and Amoebophrya) that belong to the Syndiniales, an atypical order of dinoflagellates exclu- sively composed of marine parasites. This suggests that Group II could correspond to Syndiniales, al- though this should be confirmed in the future by examining the morphology of cells from Group II. Group II appears to be abundant in coastal and oceanic ecosystems, whereas permanent anoxic waters and hy- drothermal ecosystems are usually dominated by Group I. -

Single Cell Genomics of Uncultured Marine Alveolates Shows Paraphyly of Basal Dinoflagellates
The ISME Journal (2018) 12, 304–308 © 2018 International Society for Microbial Ecology All rights reserved 1751-7362/18 www.nature.com/ismej SHORT COMMUNICATION Single cell genomics of uncultured marine alveolates shows paraphyly of basal dinoflagellates Jürgen FH Strassert1,7, Anna Karnkowska1,8, Elisabeth Hehenberger1, Javier del Campo1, Martin Kolisko1,2, Noriko Okamoto1, Fabien Burki1,7, Jan Janouškovec1,9, Camille Poirier3, Guy Leonard4, Steven J Hallam5, Thomas A Richards4, Alexandra Z Worden3, Alyson E Santoro6 and Patrick J Keeling1 1Department of Botany, University of British Columbia, Vancouver, British Columbia, Canada; 2Institute of ̌ Parasitology, Biology Centre CAS, C eské Budějovice, Czech Republic; 3Monterey Bay Aquarium Research Institute, Moss Landing, CA, USA; 4Biosciences, University of Exeter, Exeter, UK; 5Department of Microbiology and Immunology, University of British Columbia, Vancouver, British Columbia, Canada and 6Department of Ecology, Evolution and Marine Biology, University of California, Santa Barbara, CA, USA Marine alveolates (MALVs) are diverse and widespread early-branching dinoflagellates, but most knowledge of the group comes from a few cultured species that are generally not abundant in natural samples, or from diversity analyses of PCR-based environmental SSU rRNA gene sequences. To more broadly examine MALV genomes, we generated single cell genome sequences from seven individually isolated cells. Genes expected of heterotrophic eukaryotes were found, with interesting exceptions like presence of -

Science Journals
SCIENCE ADVANCES | RESEARCH ARTICLE EVOLUTIONARY BIOLOGY Copyright © 2019 The Authors, some rights reserved; An aerobic eukaryotic parasite with functional exclusive licensee American Association mitochondria that likely lacks a mitochondrial genome for the Advancement Uwe John1,2*, Yameng Lu1,3, Sylke Wohlrab1,2, Marco Groth4, Jan Janouškovec5, Gurjeet S. Kohli1,6, of Science. No claim to 1 1 7 4 1,8 original U.S. Government Felix C. Mark , Ulf Bickmeyer , Sarah Farhat , Marius Felder , Stephan Frickenhaus , Works. Distributed 9,10 11 12 7 Laure Guillou , Patrick J. Keeling , Ahmed Moustafa , Betina M. Porcel , under a Creative 1 13 Klaus Valentin , Gernot Glöckner * Commons Attribution NonCommercial Dinoflagellates are microbial eukaryotes that have exceptionally large nuclear genomes; however, their organelle License 4.0 (CC BY-NC). genomes are small and fragmented and contain fewer genes than those of other eukaryotes. The genus Amoebophrya (Syndiniales) comprises endoparasites with high genetic diversity that can infect other dinoflagellates, such as those forming harmful algal blooms (e.g., Alexandrium). We sequenced the genome (~100 Mb) of Amoebophrya ceratii to investigate the early evolution of genomic characters in dinoflagellates. The A. ceratii genome encodes almost all essential biosynthetic pathways for self-sustaining cellular metabolism, suggesting a limited dependency on its host. Although dinoflagellates are thought to have descended from a photosynthetic ancestor, A. ceratii Downloaded from appears to have completely lost its plastid and nearly all genes of plastid origin. Functional mitochondria persist in all life stages of A. ceratii, but we found no evidence for the presence of a mitochondrial genome. Instead, all mitochondrial proteins appear to be lost or encoded in the A. -

Dinophyceae Can Use Exudates As Weapons Against the Parasite Amoebophrya Sp
www.nature.com/ismecomms ARTICLE Dinophyceae can use exudates as weapons against the parasite Amoebophrya sp. (Syndiniales) ✉ Marc Long 1 , Dominique Marie2, Jeremy Szymczak2, Jordan Toullec1, Estelle Bigeard2, Marc Sourisseau1, Mickael Le Gac1, Laure Guillou2 and Cécile Jauzein1 © The Author(s) 2021 Parasites in the genus Amoebophrya sp. infest dinoflagellate hosts in marine ecosystems and can be determining factors in the demise of blooms, including toxic red tides. These parasitic protists, however, rarely cause the total collapse of Dinophyceae blooms. Experimental addition of parasite-resistant Dinophyceae (Alexandrium minutum or Scrippsiella donghaienis) or exudates into a well-established host-parasite coculture (Scrippsiella acuminata-Amoebophrya sp.) mitigated parasite success and increased the survival of the sensitive host. This effect was mediated by waterborne molecules without the need for a physical contact. The strength of the parasite defenses varied between dinoflagellate species, and strains of A. minutum and was enhanced with increasing resistant host cell concentrations. The addition of resistant strains or exudates never prevented the parasite transmission entirely. Survival time of Amoebophrya sp. free-living stages (dinospores) decreased in presence of A. minutum but not of S. donghaienis. Parasite progeny drastically decreased with both species. Integrity of the dinospore membrane was altered by A. minutum, providing a first indication on the mode of action of anti-parasitic molecules. These results demonstrate that extracellular defenses can be an effective strategy against parasites that protects not only the resistant cells producing them, but also the surrounding community. ISME Communications; (2021) 1:34 ; https://doi.org/10.1038/s43705-021-00035-x INTRODUCTION of the same species suggests a genetic determinism underlying Parasites, thought to account for half of species richness in some host specialization [18]. -

The Planktonic Protist Interactome: Where Do We Stand After a Century of Research?
The ISME Journal (2020) 14:544–559 https://doi.org/10.1038/s41396-019-0542-5 ARTICLE The planktonic protist interactome: where do we stand after a century of research? 1 1 1 1 Marit F. Markussen Bjorbækmo ● Andreas Evenstad ● Line Lieblein Røsæg ● Anders K. Krabberød ● Ramiro Logares 1,2 Received: 14 May 2019 / Revised: 17 September 2019 / Accepted: 24 September 2019 / Published online: 4 November 2019 © The Author(s) 2019. This article is published with open access Abstract Microbial interactions are crucial for Earth ecosystem function, but our knowledge about them is limited and has so far mainly existed as scattered records. Here, we have surveyed the literature involving planktonic protist interactions and gathered the information in a manually curated Protist Interaction DAtabase (PIDA). In total, we have registered ~2500 ecological interactions from ~500 publications, spanning the last 150 years. All major protistan lineages were involved in interactions as hosts, symbionts (mutualists and commensalists), parasites, predators, and/or prey. Predation was the most common interaction (39% of all records), followed by symbiosis (29%), parasitism (18%), and ‘unresolved interactions’ fi 1234567890();,: 1234567890();,: (14%, where it is uncertain whether the interaction is bene cial or antagonistic). Using bipartite networks, we found that protist predators seem to be ‘multivorous’ while parasite–host and symbiont–host interactions appear to have moderate degrees of specialization. The SAR supergroup (i.e., Stramenopiles, Alveolata, and Rhizaria) heavily dominated PIDA, and comparisons against a global-ocean molecular survey (TARA Oceans) indicated that several SAR lineages, which are abundant and diverse in the marine realm, were underrepresented among the recorded interactions.