High Risk of Sustained Ventricular Arrhythmia Recurrence After Acute Myocarditis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WPW: WOLFF-PARKINSON-WHITE Syndrome
WPW: WOLFF-PARKINSON-WHITE Syndrome What is Wolff-Parkinson-White Syndrome? Wolff-Parkinson-White Syndrome, or WPW, is named for three physicians who described a syndrome in 1930 in young people with episodes of heart racing and an abnormal pattern on their electrocardiogram (ECG or EKG). Over the next few decades, it was discovered that this ECG pattern and the heart racing was due to an extra electrical pathway in the heart. Thus, WPW is a syndrome associated with an abnormal heart rhythm, or “arrhythmia”. Most people with WPW do not have any other problems with their heart. Normally, the electrical impulses in the heart originate in the atria or top chambers of the heart and spread across the atria. The electrical impulses are then conducted to the ventricles (the pumping/bottom chambers of the heart) through a group of specialized cells called the atrioventricular node or AV node. This is usually the only electrical pathway between the atria and ventricles. In WPW, there is an additional pathway made up of a few extra cells left over from when the heart formed. The conduction of electricity through the heart causes the contractions which are the “heartbeat”. What is WPW Syndrome as opposed to a WPW ECG? A person has WPW Syndrome if they experience symptoms from abnormal conduction through the heart by the WPW pathway. Most commonly, the symptom is heart racing, or “palpitations”. The particular type of arrhythmia in WPW is called “supraventricular tachycardia” or SVT. “Tachycardia” means fast heart rate; “supraventricular” means the arrhythmia requires the cells above the ventricles to be part of the abnormal circuit. -

Cardiac Involvement in COVID-19 Patients: a Contemporary Review
Review Cardiac Involvement in COVID-19 Patients: A Contemporary Review Domenico Maria Carretta 1, Aline Maria Silva 2, Donato D’Agostino 2, Skender Topi 3, Roberto Lovero 4, Ioannis Alexandros Charitos 5,*, Angelika Elzbieta Wegierska 6, Monica Montagnani 7,† and Luigi Santacroce 6,*,† 1 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Coronary Unit and Electrophysiology/Pacing Unit, Cardio-Thoracic Department, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] 2 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Cardiac Surgery, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] (A.M.S.); [email protected] (D.D.) 3 Department of Clinical Disciplines, School of Technical Medical Sciences, University of Elbasan “A. Xhuvani”, 3001 Elbasan, Albania; [email protected] 4 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Clinical Pathology Unit, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] 5 Emergency/Urgent Department, National Poisoning Center, Riuniti University Hospital of Foggia, 71122 Foggia, Italy 6 Department of Interdisciplinary Medicine, Microbiology and Virology Unit, University of Bari “Aldo Moro”, Piazza G. Cesare, 11, 70124 Bari, Italy; [email protected] 7 Department of Biomedical Sciences and Human Oncology—Section of Pharmacology, School of Medicine, University of Bari “Aldo Moro”, Policlinico University Hospital of Bari, p.zza G. Cesare 11, 70124 Bari, Italy; [email protected] * Correspondence: [email protected] (I.A.C.); [email protected] (L.S.) † These authors equally contributed as co-last authors. Citation: Carretta, D.M.; Silva, A.M.; D’Agostino, D.; Topi, S.; Lovero, R.; Charitos, I.A.; Wegierska, A.E.; Abstract: Background: The widely variable clinical manifestations of SARS-CoV2 disease (COVID-19) Montagnani, M.; Santacroce, L. -

Non Commercial Use Only
Cardiogenetics 2017; volume 7:6304 Sudden death in a young patient with atrial fibrillation Case Report Correspondence: María Angeles Espinosa Castro, Inherited Cardiovascular Disease A 22-year-old man suffered a sudden Program, Cardiology Department, Gregorio María Tamargo, cardiac arrest without previous symptoms Marañón Hospital, Dr. Esquerdo, 46, 28007, María Ángeles Espinosa, while he was at rest, waiting for a subway Madrid, Spain. Víctor Gómez-Carrillo, Miriam Juárez, train. Cardiopulmonary resuscitation was Tel.: +34.91.586.82.90. immediately started using an Automated E-mail: [email protected] Francisco Fernández-Avilés, External Defibrillation that identified the Raquel Yotti Key words: KCNQ1; mutation; channelopa- presence of ventricular fibrillation and thy; sudden cardiac death; atrial fibrillation. Inherited Cardiovascular Disease delivered a shock. Return of spontaneous Program, Cardiology Department, circulation was achieved after three Contributions: MT, acquisition and interpreta- Gregorio Marañón Hospital, Madrid, attempts, being atrial fibrillation (AF) the tion of data for the work, ensuring that ques- Spain patient’s rhythm at this point (Figure 1). tions related to the accuracy or integrity of any He was admitted to our Cardiovascular part of the work is appropriately investigated Intensive Care Unit and therapeutic and resolved; MAE, conception of the work, hypothermia was performed over a period critical revision of the intellectual content, final approval of the version to be published, Abstract of 24 h. After completing hypothermia, ensuring that questions related to the accuracy rewarming, and another 24 h of controlled of any part of the work is appropriately inves- Sudden cardiac death (SCD) in young normothermia the patient awakened with no tigated and resolved; VG-C, acquisition and patients without structural heart disease is residual neurologic damage. -

Early Repolarization and Myocardial Scar Predict Poorest Prognosis in Patients with Coronary Artery Disease
http://dx.doi.org/10.3349/ymj.2014.55.4.928 Original Article pISSN: 0513-5796, eISSN: 1976-2437 Yonsei Med J 55(4):928-936, 2014 Early Repolarization and Myocardial Scar Predict Poorest Prognosis in Patients with Coronary Artery Disease Hye-Young Lee,1,2 Hee-Sun Mun,1 Jin Wi,1 Jae-Sun Uhm,1 Jaemin Shim,1 Jong-Youn Kim,1 Hui-Nam Pak,1 Moon-Hyoung Lee,1 and Boyoung Joung1 1Division of Cardiology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul; 2Division of Cardiology, Department of Internal Medicine, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea. Received: July 2, 2013 Purpose: Recent studies show positive association of early repolarization (ER) Revised: October 27, 2013 with the risk of life-threatening arrhythmias in patients with coronary artery dis- Accepted: November 4, 2013 ease (CAD). This study was to investigate the relationships of ER with myocardial Corresponding author: Dr. Boyoung Joung, scarring and prognosis in patients with CAD. Materials and Methods: Of 570 Division of Cardiology, consecutive CAD patients, patients with and without ER were assigned to ER Department of Internal Medicine, Yonsei University College of Medicine, group (n=139) and no ER group (n=431), respectively. Myocardial scar was eval- 50-1 Yonsei-ro, Seodaemun-gu, uated using cardiac single-photon emission computed tomography. Results: ER Seoul 120-752, Korea. group had previous history of myocardial infarction (33% vs. 15%, p<0.001) and Tel: 82-2-2228-8460, Fax: 82-2-393-2041 lower left ventricular ejection fraction (57±13% vs. -

Myocarditis and Cardiomyopathy
CE: Tripti; HCO/330310; Total nos of Pages: 6; HCO 330310 REVIEW CURRENT OPINION Myocarditis and cardiomyopathy Jonathan Buggey and Chantal A. ElAmm Purpose of review The aim of this study is to summarize the literature describing the pathogenesis, diagnosis and management of cardiomyopathy related to myocarditis. Recent findings Myocarditis has a variety of causes and a heterogeneous clinical presentation with potentially life- threatening complications. About one-third of patients will develop a dilated cardiomyopathy and the pathogenesis is a multiphase, mutlicompartment process that involves immune activation, including innate immune system triggered proinflammatory cytokines and autoantibodies. In recent years, diagnosis has been aided by advancements in cardiac MRI, and in particular T1 and T2 mapping sequences. In certain clinical situations, endomyocardial biopsy (EMB) should be performed, with consideration of left ventricular sampling, for an accurate diagnosis that may aid treatment and prognostication. Summary Although overall myocarditis accounts for a minority of cardiomyopathy and heart failure presentations, the clinical presentation is variable and the pathophysiology of myocardial damage is unique. Cardiac MRI has significantly improved diagnostic abilities, but endomyocardial biopsy remains the gold standard. However, current treatment strategies are still focused on routine heart failure pharmacotherapies and supportive care or cardiac transplantation/mechanical support for those with end-stage heart failure. Keywords cardiac MRI, cardiomyopathy, endomyocardial biopsy, myocarditis INTRODUCTION prevalence seen in children and young adults aged Myocarditis refers to inflammation of the myocar- 20–30 years [1]. dium and may be caused by infectious agents, systemic diseases, drugs and toxins, with viral infec- CAUSE tions remaining the most common cause in the developed countries [1]. -

2015 ESC Guidelines for the Management of Patients
European Heart Journal Advance Access published August 29, 2015 European Heart Journal ESC GUIDELINES doi:10.1093/eurheartj/ehv316 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Downloaded from Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) http://eurheartj.oxfordjournals.org/ Authors/Task Force Members: Silvia G. Priori* (Chairperson) (Italy), Carina Blomstro¨ m-Lundqvist* (Co-chairperson) (Sweden), Andrea Mazzanti† (Italy), Nico Bloma (The Netherlands), Martin Borggrefe (Germany), John Camm (UK), Perry Mark Elliott (UK), Donna Fitzsimons (UK), Robert Hatala (Slovakia), Gerhard Hindricks (Germany), Paulus Kirchhof (UK/Germany), Keld Kjeldsen (Denmark), Karl-Heinz Kuck (Germany), Antonio Hernandez-Madrid (Spain), Nikolaos Nikolaou (Greece), Tone M. Norekva˚l (Norway), Christian Spaulding by guest on October 21, 2015 (France), and Dirk J. Van Veldhuisen (The Netherlands) * Corresponding authors: Silvia Giuliana Priori, Department of Molecular Medicine University of Pavia, Cardiology & Molecular Cardiology, IRCCS Fondazione Salvatore Maugeri, Via Salvatore Maugeri 10/10A, IT-27100 Pavia, Italy, Tel: +39 0382 592 040, Fax: +39 0382 592 059, Email: [email protected] Carina Blomstro¨m-Lundqvist, Department of Cardiology, Institution of Medical Science, Uppsala University, SE-751 85 Uppsala, Sweden, Tel: +46 18 611 3113, Fax: +46 18 510 243, Email: [email protected] aRepresenting the Association for European Paediatric and Congenital Cardiology (AEPC). †Andrea Mazzanti: Coordinator, affiliation listed in the Appendix. ESC Committee for Practice Guidelines (CPG) and National Cardiac Societies document reviewers: listed in the Appendix. -

Myocarditis, Pericarditis and Other Pericardial Diseases
Heart 2000;84:449–454 Diagnosis is easiest during epidemics of cox- GENERAL CARDIOLOGY sackie infections but diYcult in isolated cases. Heart: first published as 10.1136/heart.84.4.449 on 1 October 2000. Downloaded from These are not seen by cardiologists unless they develop arrhythmia, collapse or suVer chest Myocarditis, pericarditis and other pain, the majority being dealt with in the primary care system. pericardial diseases Acute onset of chest pain is usual and may mimic myocardial infarction or be associated 449 Celia M Oakley with pericarditis. Arrhythmias or conduction Imperial College School of Medicine, Hammersmith Hospital, disturbances may be life threatening despite London, UK only mild focal injury, whereas more wide- spread inflammation is necessary before car- diac dysfunction is suYcient to cause symp- his article discusses the diagnosis and toms. management of myocarditis and peri- Tcarditis (both acute and recurrent), as Investigations well as other pericardial diseases. The ECG may show sinus tachycardia, focal or generalised abnormality, ST segment eleva- tion, fascicular blocks or atrioventricular con- Myocarditis duction disturbances. Although the ECG abnormalities are non-specific, the ECG has Myocarditis is the term used to indicate acute the virtue of drawing attention to the heart and infective, toxic or autoimmune inflammation of leading to echocardiographic and other investi- the heart. Reversible toxic myocarditis occurs gations. Echocardiography may reveal segmen- in diphtheria and sometimes in infective endo- -
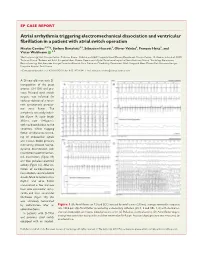
Atrial Arrhythmia Triggering Electromechanical Dissociation And
EP CASE REPORT ....................................................................................................................................................... Atrial arrhythmia triggering electromechanical dissociation and ventricular fibrillation in a patient with atrial switch operation Nicolas Combes1,2,3*, Stefano Bartoletti1,2,Se´bastien Hascoet€ 3, Olivier Vahdat2, Franc¸ois Heitz2, and Victor Waldmann 4,5 1Electrophysiology Unit, Clinique Pasteur, Toulouse, France; 2Pediatric and Adult Congenital Heart Disease Department, Clinique Pasteur, 45, Avenue de Lombez, 31076 Toulouse, France; 3Pediatric and Adult Congenital Heart Disease Department, Hoˆpital Marie Lannelongue, Le Plessis-Robinson, France; 4Cardiology Department, Electrophysiology Unit, European Georges Pompidou Hospital, Paris, France; and 5Cardiology Department, Adult Congenital Heart Disease Unit, European Georges Pompidou Hospital, Paris, France * Corresponding author. Tel: 133 562213131; fax: 133 562211641. E-mail address: [email protected] A 26-year-old man with D- transposition of the great arteries (D-TGA) and pre- vious Mustard atrial switch surgery was referred for catheter ablation of a recur- rent symptomatic paroxys- mal atrial flutter. The arrhythmia was easily induci- ble (Figure 1A, cycle length 310 ms, rate 194 b.p.m.), with rapid conduction to the ventricles. While mapping flutter, simultaneous record- ing of endocardial signals and invasive blood pressure monitoring showed haemo- dynamic deterioration with intermittent electromechan- ical dissociation (Figure 1B) and then pulseless electrical activity (Figure 1C). After ini- tiation of cardiopulmonary resuscitation, several electric shocks failed to restore sinus rhythm and atrial flutter transitioned a few minutes later into ventricular tachy- cardia and then ventricular fibrillation (Figure 1D); this was ultimately terminated by defibrillation after an Figure 1 (A) Atrial flutter on 12-lead ECG induced by atrial bursts (240 ms), average ventricular response adrenaline bolus. -

Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: the Role of Multimodality Imaging to Detect High-Risk Features
diagnostics Review Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features Anna Giulia Pavon 1,2,*, Pierre Monney 1,2,3 and Juerg Schwitter 1,2,3 1 Cardiac MR Center (CRMC), Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland; [email protected] (P.M.); [email protected] (J.S.) 2 Cardiovascular Department, Division of Cardiology, Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland 3 Faculty of Biology and Medicine, University of Lausanne (UniL), 1100 Lausanne, Switzerland * Correspondence: [email protected]; Tel.: +41-775-566-983 Abstract: Mitral valve prolapse (MVP) was first described in the 1960s, and it is usually a benign condition. However, a subtype of patients are known to have a higher incidence of ventricular arrhythmias and sudden cardiac death, the so called “arrhythmic MVP.” In recent years, several studies have been published to identify the most important clinical features to distinguish the benign form from the potentially lethal one in order to personalize patient’s treatment and follow-up. In this review, we specifically focused on red flags for increased arrhythmic risk to whom the cardiologist must be aware of while performing a cardiovascular imaging evaluation in patients with MVP. Keywords: mitral valve prolapse; arrhythmias; cardiovascular magnetic resonance Citation: Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality 1. Mitral Valve and Arrhythmias: A Long Story Short Imaging to Detect High-Risk Features. In the recent years, the scientific community has begun to pay increasing attention Diagnostics 2021, 11, 683. -

Antithrombotic Therapy in Atrial Fibrillation Associated with Valvular Heart Disease
Europace (2017) 0, 1–21 EHRA CONSENSUS DOCUMENT doi:10.1093/europace/eux240 Antithrombotic therapy in atrial fibrillation associated with valvular heart disease: a joint consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology Working Group on Thrombosis, endorsed by the ESC Working Group on Valvular Heart Disease, Cardiac Arrhythmia Society of Southern Africa (CASSA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), South African Heart (SA Heart) Association and Sociedad Latinoamericana de Estimulacion Cardıaca y Electrofisiologıa (SOLEACE) Gregory Y. H. Lip1*, Jean Philippe Collet2, Raffaele de Caterina3, Laurent Fauchier4, Deirdre A. Lane5, Torben B. Larsen6, Francisco Marin7, Joao Morais8, Calambur Narasimhan9, Brian Olshansky10, Luc Pierard11, Tatjana Potpara12, Nizal Sarrafzadegan13, Karen Sliwa14, Gonzalo Varela15, Gemma Vilahur16, Thomas Weiss17, Giuseppe Boriani18 and Bianca Rocca19 Document Reviewers: Bulent Gorenek20 (Reviewer Coordinator), Irina Savelieva21, Christian Sticherling22, Gulmira Kudaiberdieva23, Tze-Fan Chao24, Francesco Violi25, Mohan Nair26, Leandro Zimerman27, Jonathan Piccini28, Robert Storey29, Sigrun Halvorsen30, Diana Gorog31, Andrea Rubboli32, Ashley Chin33 and Robert Scott-Millar34 * Corresponding author. Tel/fax: þ44 121 5075503. E-mail address: [email protected] Published on behalf of the European Society of Cardiology. All rights reserved. VC The Author 2017. For permissions, please email: [email protected]. 2 G.Y.H. Lip 1Institute of Cardiovascular Sciences, University of Birmingham and Aalborg Thrombosis Research Unit, Department of Clinical Medicine, Aalborg University, Denmark (Chair, representing EHRA); 2Sorbonne Universite´ Paris 6, ACTION Study Group, Institut De Cardiologie, Groupe Hoˆpital Pitie´-Salpetrie`re (APHP), INSERM UMRS 1166, Paris, France; 3Institute of Cardiology, ‘G. -

Constrictive Pericarditis Causing Ventricular Tachycardia.Pdf
EP CASE REPORT ....................................................................................................................................................... A visually striking calcific band causing monomorphic ventricular tachycardia as a first presentation of constrictive pericarditis Kian Sabzevari 1*, Eva Sammut2, and Palash Barman1 1Bristol Heart Institute, UH Bristol NHS Trust UK, UK; and 2Bristol Heart Institute, UH Bristol NHS Trust UK & University of Bristol, UK * Corresponding author. Tel: 447794900287; fax: 441173425926. E-mail address: [email protected] Introduction Constrictive pericarditis (CP) is a rare condition caused by thickening and stiffening of the pericar- dium manifesting in dia- stolic dysfunction and enhanced interventricu- lar dependence. In the developed world, most cases are idiopathic or are associated with pre- vious cardiac surgery or irradiation. Tuberculosis remains a leading cause in developing areas.1 Most commonly, CP presents with symptoms of heart failure and chest discomfort. Atrial arrhythmias have been described as a rare pre- sentation, but arrhyth- mias of ventricular origin have not been reported. Figure 1 (A) The 12 lead electrocardiogram during sustained ventricular tachycardia is shown; (B and C) Case report Different projections of three-dimensional reconstructions of cardiac computed tomography demonstrating a A 49-year-old man with a striking band of calcification around the annulus; (D) Carto 3DVR mapping—the left hand panel (i) demonstrates a background of diabetes, sinus beat with late potentials at the point of ablation in the coronary sinus, the right hand panel (iii) shows the hypertension, and hyper- pacemap with a 89% match to the clinical tachycardia [matching the morphology seen on 12 lead ECG (A)], and cholesterolaemia and a the middle panel (ii) displays the three-dimensional voltage map. -

J Wave Syndromes
Review Article http://dx.doi.org/10.4070/kcj.2016.46.5.601 Print ISSN 1738-5520 • On-line ISSN 1738-5555 Korean Circulation Journal J Wave Syndromes: History and Current Controversies Tong Liu, MD1, Jifeng Zheng, MD2, and Gan-Xin Yan, MD3,4 1Tianjin Key Laboratory of Ionic-Molecular Function of Cardiovascular disease, Department of Cardiology, Tianjin Institute of Cardiology, The Second Hospital of Tianjin Medical University, Tianjin, 2Department of cardiology, The Second Hospital of Jiaxing, Jiaxing, China, 3Lankenau Institute for Medical Research and Lankenau Medical Center, Wynnewood, Pennsylvania, USA, 4The First Affiliated Hospital, Medical School of Xi'an Jiaotong University, Xi'an, China The concept of J wave syndromes was first proposed in 2004 by Yan et al for a spectrum of electrocardiographic (ECG) manifestations of prominent J waves that are associated with a potential to predispose affected individuals to ventricular fibrillation (VF). Although the concept of J wave syndromes is widely used and accepted, there has been tremendous debate over the definition of J wave, its ionic and cellular basis and arrhythmogenic mechanism. In this review article, we attempted to discuss the history from which the concept of J wave syndromes (JWS) is evolved and current controversies in JWS. (Korean Circ J 2016;46(5):601-609) KEY WORDS: Brugada syndrome; Sudden cardiac death; Ventricular fibrillation. Introduction History of J wave and J wave syndromes The concept of J wave syndromes was first proposed in 2004 The J wave is a positive deflection seen at the end of the QRS by Yan et al.1) for a spectrum of electrocardiographic (ECG) complex; it may stand as a distinct “delta” wave following the QRS, manifestations of prominent J waves that are associated with a or be partially buried inside the QRS as QRS notching or slurring.