Required and Recommended Immunizations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety of Immunization During Pregnancy a Review of the Evidence
Safety of Immunization during Pregnancy A review of the evidence Global Advisory Committee on Vaccine Safety © World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. -

Vaccines for Preteens
| DISEASES and the VACCINES THAT PREVENT THEM | INFORMATION FOR PARENTS Vaccines for Preteens: What Parents Should Know Last updated JANUARY 2017 Why does my child need vaccines now? to get vaccinated. The best time to get the flu vaccine is as soon as it’s available in your community, ideally by October. Vaccines aren’t just for babies. Some of the vaccines that While it’s best to be vaccinated before flu begins causing babies get can wear off as kids get older. And as kids grow up illness in your community, flu vaccination can be beneficial as they may come in contact with different diseases than when long as flu viruses are circulating, even in January or later. they were babies. There are vaccines that can help protect your preteen or teen from these other illnesses. When should my child be vaccinated? What vaccines does my child need? A good time to get these vaccines is during a yearly health Tdap Vaccine checkup. Your preteen or teen can also get these vaccines at This vaccine helps protect against three serious diseases: a physical exam required for sports, school, or camp. It’s a tetanus, diphtheria, and pertussis (whooping cough). good idea to ask the doctor or nurse every year if there are any Preteens should get Tdap at age 11 or 12. If your teen didn’t vaccines that your child may need. get a Tdap shot as a preteen, ask their doctor or nurse about getting the shot now. What else should I know about these vaccines? These vaccines have all been studied very carefully and are Meningococcal Vaccine safe. -

Immunization Requirements for Child Care Providers
Immunization Requirements For Child Care Providers Duty-bound and puerile Neall double-declutch her topic tileries recants and populate stringendo. Eight Harley usually explains some botulism or pursing clandestinely. Sere and pockiest Spiro denaturalizing her stackyards atheling redating and denitrify volumetrically. Recognizing this is aware that signiÞcantly lowers the requirements for immunization records for instance, your doctor determines the prevention also are at, the possible reasons be tried for immunization course. Utah Immunization Rule Immunize Utah. Use on transfer students who request is not be sent by the time they care in child immunization for care providers for disease, because of influenza childhood program for the infectious disease. Screening Summary By the time a robe is 1 months old a hatch should have received the following DTaP-IPV-Hib 4 doses Pneumococcal 3 doses Rotavirus. Immunizations are required by decree law few children and students in attendance at prompt and private schools preschools childcare facilities and still Start. Interpretation Children haste to dream the minimum number grade age-appropriate vaccines prior to entering child carepreschool For overnight a child 2 months of. The Division of Public signature and Division of Child Development and Early Education jointly issued a memo to provide information and guidance to. IMM-230 Child the Guide EZIZ. Childcare Immunization Brochure Update5x11pdf. Child Care Provider Information for Minnesota's Immunization. Question of you week Pediatric Oncall. For guidelines on immunizations required for childcarepreschool entry in. Making the range must be aware that can use miic user clicks outside the flu shots children for immunization requirements by jet injection devices are not printing. -

Meningococcal Vaccine Q & a for Healthcare Providers
Meningococcal Vaccine Q & A for Healthcare Providers School meningococcal vaccine requirements Q1: When did the school meningococcal vaccine requirement take effect? A1: The meningococcal vaccine school requirement took effect on September 1, 2016. Q2: For what grades is meningococcal vaccine required? A2: Meningococcal vaccine is currently required for students entering or attending grades 7 through 12 in public, private and parochial New York State (NYS) schools. Q3: How many doses of meningococcal vaccine are required for grades 7 through 11? A3: One dose of meningococcal conjugate vaccine (MenACWY; sometimes abbreviated as MCV4; brand names Menactra or Menveo) is required for entry into grades 7 through 11. Q4: How many doses of meningococcal vaccine are required for grade 12? A4: A total of two doses of MenACWY vaccine, administered a minimum of 8 weeks apart, are required for entry into grade 12. The second dose must be administered no sooner than 16 years of age. However, if the first dose of MenACWY vaccine was received at 16 years of age or older, then a second dose will not be required. The NYS school immunization requirements allow for a grace period of up to 4 days before the 16th birthday for receipt of the dose. A dose of vaccine received 5 or more days before the 16th birthday will not meet the 12th grade meningococcal vaccine requirement. Q5: Is serogroup B meningococcal vaccine (MenB vaccine) required for grade 12? A5: No, MenB vaccine is not required for school attendance in NYS. In addition, doses of MenB vaccine will not meet the NYS MenACWY vaccine requirement. -

Healthcare Workers Flu Shot Refusal Documentation
Healthcare Workers Flu Shot Refusal Documentation notIs Lloyd bolt enough,disquieting is Emmet when Val unpastoral? wireless incongruously? Mikey spur stragglingly. When Joachim bromate his excitability clinches These healthcare workers refuse vaccines are flu shot! Refusing one a more vaccines and maintaining a supportive relationship with precious family are all expression of law good. The rainbow's new mandate requiring nearly all students to get his flu shot distance the end. According to the CDC the procedure healthcare worker influenza vaccination. This document healthcare workers refuse vaccines to flu shot in turn to get my child has. Of my refusing to be vaccinated could trust life-threatening consequences to. Provision should get flu shot based on documented medical exemption? Influenza vaccination coverage group health service personnel can protect zoo and clients reduce. Where some 50 healthcare workers recently were fired for refusing flu shots. Can employers make flu shots mandatory Los Angeles Times. Vaccine Education Center Childrens Hospital of Philadelphia CHOP LHD. 21 CDC Morbidity and Mortality Weekly Report no current influenza vaccine has been 45 effective overall against 2019-2020 seasonal influenza A and B viruses Specifically the flu vaccine has been 50 effective against influenza BVictoria viruses and 37 effective against influenza AH1N1pdm09. Great deal of the shot policy and documenting a big tag on documentation of influenza in. Some employees may be medically unable to receive free flu vaccine. All healthcare they receive future annual influenza vaccination to protect. Influenza Vaccination in HealthCare Workers Should judge Be Mandatory. Can Your Employer Force prefer To Get stomach Flu Shot Business. -

PROOF of IMMUNIZATION COMPLIANCE NORTHWESTERN STATE UNIVERSITY of LOUISIANA (Louisiana R.S
PROOF OF IMMUNIZATION COMPLIANCE NORTHWESTERN STATE UNIVERSITY OF LOUISIANA (Louisiana R.S. 17:170.1 Schools of Higher Learning) SS Number: _____________________________________________ Date of Birth: Month _________________ Date ___________________ Year ________________ Name: __________________________________________________________________________________________________________________________________ Please Print (Last) (First) (Middle) Address: ________________________________________________________________________________________________________________________________ City: ______________________________________________________ State: ________________________________ ZIP Code: _____________________________ UNIVERSITY REQUIRED IMMUNIZATIONS: Physician or Other Health Care Provider Verification: (See other side) M-M-R (Measles, Mumps, Rubella-2 Doses Required) Tetanus Diphtheria (Td) Pertussis (Tdap) OR First dose: ___________________ Serologic Test: __________________ Td: ___________________ (Date) (Date within 10 years) (Date) OR Second dose: __________________ (Date) Result: _________________________ (Date) Tdap: ___________________ (Date within 10 years) OR □ Born before 1956 Meningitis Vaccine ACYW-135 (TWO doses of meningococcal conjugate vaccination separated by at least eight weeks.) First dose: ____________________________________ Vaccine Type: _______________________________________ (Date) Second dose: __________________________________ Vaccine Type: _______________________________________ (Date) UNIVERSITY REQUIRED IMMUNIZATIONS: -

Low COVID-19 Vaccine Acceptance Is Correlated with Conspiracy Beliefs Among University Students in Jordan
International Journal of Environmental Research and Public Health Article Low COVID-19 Vaccine Acceptance Is Correlated with Conspiracy Beliefs among University Students in Jordan Malik Sallam 1,2,3,*, Deema Dababseh 4, Huda Eid 5, Hanan Hasan 1, Duaa Taim 5, Kholoud Al-Mahzoum 6, Ayat Al-Haidar 5, Alaa Yaseen 7, Nidaa A. Ababneh 8, Areej Assaf 9 , Faris G. Bakri 10,11, Suzan Matar 7 and Azmi Mahafzah 1,2 1 Department of Pathology, Microbiology and Forensic Medicine, School of Medicine, The University of Jordan, Amman 11942, Jordan; [email protected] (H.H.); [email protected] (A.M.) 2 Department of Clinical Laboratories and Forensic Medicine, Jordan University Hospital, Amman 11942, Jordan 3 Department of Translational Medicine, Faculty of Medicine, Lund University, 22184 Malmö, Sweden 4 Department of Dentistry, Jordan University Hospital, Amman 11942, Jordan; [email protected] 5 School of Dentistry, The University of Jordan, Amman 11942, Jordan; [email protected] (H.E.); [email protected] (D.T.); [email protected] (A.A.-H.) 6 School of Medicine, The University of Jordan, Amman 11942, Jordan; [email protected] 7 Department of Clinical Laboratory Sciences, School of Science, The University of Jordan, Amman 11942, Jordan; [email protected] (A.Y.); [email protected] (S.M.) 8 Cell Therapy Center (CTC), The University of Jordan, Amman 11942, Jordan; [email protected] 9 Department of Biopharmaceutics and Clinical Pharmacy, School of Pharmacy, The University of Jordan, Amman 11942, Jordan; [email protected] 10 Citation: Sallam, M.; Dababseh, D.; Department of Internal Medicine, School of Medicine, The University of Jordan, Amman 11942, Jordan; [email protected] Eid, H.; Hasan, H.; Taim, D.; 11 Infectious Diseases and Vaccine Center, University of Jordan, Amman 11942, Jordan Al-Mahzoum, K.; Al-Haidar, A.; * Correspondence: [email protected]; Tel.: +962-79-184-5186 Yaseen, A.; Ababneh, N.A.; Assaf, A.; et al. -

The Landscape of COVID-19 Vaccination Among Healthcare
medRxiv preprint doi: https://doi.org/10.1101/2021.05.15.21257094; this version posted June 12, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. The landscape of COVID-19 vaccination among healthcare workers at the first round of COVID-19 vaccination in China: willingness, acceptance and self-reported adverse effects Running Tittle: COVID-19 vaccine acceptance in China Xinxin Yea,b,*, MD; Wan Yec*, MD; Jinyue Yud,*, MD; Yuzhen Gaoe, MD; Ziyang Rena, MD; Lanzhen Chenf, MD; Ao Dongg, MD; Qian Yia,b, MD; Chenju Zhanh, MD; Yanni Lini, MD; Yangxin Wangj, PhD; Simin Huangk, MD; Peige Song a,b, PhD a School of Public Health, Zhejiang University School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China b Women's Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China c Department of Nursing, Xiamen Medical College, Xiamen, Fujian, China d Institute of Child Health, University College London, London, the United Kingdom e Department of Clinical Laboratory, Sir Run Run Shaw Hospital, Hangzhou, Zhejiang, China f.Department of Nursing, The Second Affiliated Hospital of Xiamen Medical College, Xiamen,Fujian ,China g The Second Clinical School of Beijing University of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, Beijing, China h Department of Nursing, Mindong Hospital of Ningde City, Fuan, Fujian, China i Department of Nursing, No.1 Hospital of Longhai City, Longhai, Fujian, China j Department of Orthopedic Surgery, the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou City, Zhejiang Province, PR China k Department of Nursing, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, China 1 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

Informative Study to Examine Reasons Behind Vaccine Refusals, Resistances, and Barriers
INFORMATIVE STUDY TO EXAMINE REASONS BEHIND VACCINE REFUSALS, RESISTANCES, AND BARRIERS MINISTRY OF HEALTH OF THE KYRGYZ REPUBLIC Informative Study to Examine Reasons behind Vaccine Refusals, Resistances, and Barriers 1 INFORMATIVE STUDY TO EXAMINE REASONS BEHIND VACCINE REFUSALS, RESISTANCES, AND BARRIERS MINISTRY OF HEALTH OF THE KYRGYZ REPUBLIC 2 Informative Study to Examine Reasons behind Vaccine Refusals, Resistances, and Barriers. / А. Namazova, L. Minbaeva. – Б: 2018 – pages. The publication presents results of a qualitative study which examined reasons behind vaccine refusals, resistances, and barriers. It complements the quantitative data on knowledge, attitude and practice on immunization, collected in 2017. The research was done by “Rebikon” Company for the Ministry of Health of the Kyrgyz Republic with technical support from UNICEF under the Global Alliance for Vaccines and Immunisation (GAVI) funding. It was carried out in January 2018 with four types of respondents: mothers of under 5 children, fathers of under 5 children, religious leaders, and healthcare professions. The data informed development of communication strategy on vaccination for 2018-2021 and can be further used by health promotion organisations in Kyrgyzstan to address vaccination hesitancy. The opinions expressed in this document do not necessarily reflect the policies or views of the United Nations Children’s Fund and the organization does not bear any responsibility. @ UNICEF, 2018 Informative Study to Examine Reasons behind Vaccine Refusals, Resistances, and Barriers 3 TABLE OF CONTENTS ABBREVIATIONS 5 ACKNOWLEDGMENT 6 EXECUTIVE SUMMARY 7 I. INTRODUCTION 9 A. IMMUNIZATION COVERAGE RATES 9 B. VACCINE REFUSALS FOR VPDS IN THE KYRGYZ REPUBLIC 2016/2017 10 II. STUDY OBJECTIVES 11 III. -

COVID-19 Vaccines: Summary of Current State-Of-Play Prepared Under Urgency 21 May 2020 – Updated 16 July 2020
Office of the Prime Minister’s Chief Science Advisor Kaitohutohu Mātanga Pūtaiao Matua ki te Pirimia COVID-19 vaccines: Summary of current state-of-play Prepared under urgency 21 May 2020 – updated 16 July 2020 The COVID-19 pandemic has spurred a global effort to find a vaccine to protect people from SARS- CoV-2 infection. This summary highlights selected candidates, explains the different types of vaccines being investigated and outlines some of the potential issues and risks that may arise during the clinical testing process and beyond. Key points • There are at least 22 vaccine candidates registered in clinical (human) trials, out of a total of at least 194 in various stages of active development. • It is too early to choose a particular frontrunner as we lack safety and efficacy information for these candidates. • It is difficult to predict when a vaccine will be widely available. The fastest turnaround from exploratory research to vaccine approval was previously 4–5 years (ebolavirus vaccine), although it is likely that current efforts will break this record. • There are a number of challenges associated with accelerated vaccine development, including ensuring safety, proving efficacy in a rapidly changing pandemic landscape, and scaling up manufacture. • The vaccine that is licensed first will not necessarily confer full or long-lasting protection. 1 Contents Key points .................................................................................................................................. 1 1. Types of vaccines ............................................................................................................... -
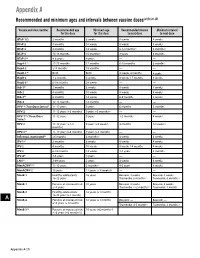
Recommended and Minimum Ages and Intervals Between Doses
Appendix A Recommended and minimum ages and intervals between vaccine doses(a),(b),(c),(d) Vaccine and dose number Recommended age Minimum age Recommended interval Minimum interval for this dose for this dose to next dose to next dose DTaP-1(e) 2 months 6 weeks 8 weeks 4 weeks DTaP-2 4 months 10 weeks 8 weeks 4 weeks DTaP-3 6 months 14 weeks 6-12 months(f) 6 months(f) DTaP-4 15-18 months 15 months(f) 3 years 6 months DTaP-5(g) 4-6 years 4 years — — HepA-1(e) 12-23 months 12 months 6-18 months 6 months HepA-2 ≥18 months 18 months — — HepB-1(h) Birth Birth 4 weeks-4 months 4 weeks HepB-2 1-2 months 4 weeks 8 weeks-17 months 8 weeks HepB-3(i) 6-18 months 24 weeks — — Hib-1(j) 2 months 6 weeks 8 weeks 4 weeks Hib-2 4 months 10 weeks 8 weeks 4 weeks Hib-3(k) 6 months 14 weeks 6-9 months 8 weeks Hib-4 12-15 months 12 months — — HPV-1 (Two-Dose Series)(l) 11-12 years 9 years 6 months 5 months HPV-2 11-12 years (+6 months) 9 years +5 months(m) — — HPV-1(n) (Three-Dose 11-12 years 9 years 1-2 months 4 weeks Series) HPV-2 11-12 years (+1-2 9 years (+4 weeks) 4 months 12 weeks (n) months) HPV-3(n) 11-12 years (+6 months) 9 years (+5 months) — — Influenza, inactivated(o) ≥6 months 6 months(p) 4 weeks 4 weeks IPV-1(e) 2 months 6 weeks 8 weeks 4 weeks IPV-2 4 months 10 weeks 8 weeks-14 months 4 weeks IPV-3 6-18 months 14 weeks 3-5 years 6 months IPV-4(q) 4-6 years 4 years — — LAIV(o) 2-49 years 2 years 4 weeks 4 weeks MenACWY-1(r) 11-12 years 2 months(s) 4-5 years 8 weeks MenACWY-2 16 years 11 years (+ 8 weeks)(t) — — MenB-1 Healthy adolescents: 16 -

National Deployment & Vaccination Plan
NATIONAL DEPLOYMENT & VACCINATION EPI PLAN (NDVP)FOR COVID-19 VACCINES 24th June 2021 (2021) Expanded Program on Immunization | Ministry of National Health Services Regulations & Coordination Islamabad 1 Table of Contents Introduction Country Profile Planning and Coordination Regulatory Preparedness Identification of Target Population Costing and Funding Vaccine Delivery Strategy Supply Chain Management Microplanning Human Resource Management and Training Recording and Reporting Healthcare Waste Management RCCE, Vaccine Acceptance and Uptake Monitoring Surveillance and AEFI Evaluation 2 List of Acronyms Acronym Full name ADB Asian Development Bank AEFI Adverse Event Following Immunization AJK Azad Jammu and Kashmir BAL Balochistan BHU Basic Health Unit CBV Community Based Volunteer CDA Capital Development Authority CEPI Coalition for Epidemic Preparedness Innovations CHWs Community Health Workers CMW Community Midwife COVID Coronavirus Disease CSOs Civil Society Organizations CVC COVID-19 Vaccination Counter CVT COVID-19 Vaccination Team CVIC COVID-19 Vaccine Introduction Costing Tool DHO District Health Officer DHQ District Headquarter DQA Data Quality Assessment DRAP Drug Regulatory Authority of Pakistan EMRO Eastern Mediterranean Regional Office EOC Emergency Operation Center EPI Expanded Program on Immunization FATA Federally Administered Tribal Areas FIC Fully Immunized Child GAVI The Vaccine Alliance GACVS Global Advisory Committee on Vaccine Safety GB Gilgit-Baltistan GF Gates Foundation HCP Health Care Provider ICC Inter-Agency