The Catalog of Human Cytokeratins: Patterns of Expression in Normal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Situlocalization of Cytoskeletal Elements in the Human Trabecular
Investigative Ophthalmology & Visual Science. Vol. 31. No. 9. September 1990 Cops right £• Association lor Research in Vision and Ophthalmology In Situ Localization of Cytoskeletal Elements in the Human Trabecular Meshwork and Cornea Robert N. Weinreb* and Mark I. Ryderf The authors compared cytoskeletal elements of the in situ human trabccular-mcshwork cell with in situ human corneal cells using indirect immunofluorcsccncc staining for tubulin and intermediate filaments (vimentin, cytokeratin, and desmin) and NBD-phallacidin staining for f-actin using both fixed frozen and unfixed frozen sections from postmortem eyes. Both f-actin and tubulin were found throughout the cell body of trabecular-meshwork cells, keratocytes, corneal endothelium, and corneal epithelium. The f-actin staining pattern was concentrated at the cell periphery of these four cell types. Vimentin stain was intensely localized in focal areas of the trabecular-meshwork cell, keratocytes, and throughout the corneal cndothelium. A general anticytokeratin antibody was intensely localized in corneal epithelium and endothelium. However, PKK-1 anticytokeratin antibody was seen only in superficial layers of corneal epithelium and not in corneal endothelium. The 4.62 anticytokeratin antibody was not observed in either corneal epithelium or endothelium. None of these three cytokera- tin antibodies were seen in trabccular-mcshwork cells or keratocytes. Desmin stain was not noted in any of these cell types. In general, cytoskeletal staining of unfixed frozen sections showed a similar staining pattern for f-actin and tubulin but a more uniform and intense staining pattern for vimentin and cytokcratin compared with fixed frozen material. The authors conclude that these cytoskclctal stains can differentiate human Irabeciilar-meshwork cells from cells of the cornea in situ. -

Decreased Expression of Profilin 2 in Oral Squamous Cell Carcinoma and Its Clinicopathological Implications
ONCOLOGY REPORTS 26: 813-823, 2011 Decreased expression of profilin 2 in oral squamous cell carcinoma and its clinicopathological implications C.Y. MA1,2, C.P. ZHANG1,2, L.P. ZHONG1,2, H.Y. PAN1,2, W.T. CHEN1,2, L.Z. WANG3, O.W. ANDREW4, T. JI1 and W. HAN1,2 1Department of Oral and Maxillofacial Surgery, Ninth People's Hospital, College of Stomatology; 2Shanghai Key Laboratory of Stomatology and Shanghai Research Institute of Stomatology; 3Department of Oral Pathology, Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, P.R. China; 4Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, National University of Singapore, Singapore 119074, Singapore Received February 8, 2011; Accepted April 11, 2011 DOI: 10.3892/or.2011.1365 Abstract. Profilins are small proteins essential for many clinical and pathological significance. In conclusion, PFN2 normal cellular dynamics and constitute one of the crucial can be utilized as both a potential suppressor marker and a components of actin-based cellular motility. Several recent prognostic protein for OSCC. The function of PFN2 may be to studies have implicated a role for the profilin (PFN) family in regulate the N-WASP/Arp2/3 signaling pathway. cancer pathogenesis and progression. However, their expression and promising functions are largely unknown in oral squamous Introduction cell carcinoma (OSCC). In this study, we analyzed the correlation between PFN1 and PFN2 expression in vitro and Oral squamous cell carcinoma (OSCC) is a significant public in vivo. The protein expression levels were roughly compared health problem with >300,000 new cases being diagnosed between cell lines (HIOEC, HB96) with the employment of annually worldwide (1). -

A Significant Soluble Keratin Fraction In
Journal of Cell Science 105, 433-444 (1993) 433 Printed in Great Britain © The Company of Biologists Limited 1993 A significant soluble keratin fraction in ‘simple’ epithelial cells Lack of an apparent phosphorylation and glycosylation role in keratin solubility Chih-Fong Chou*, Carrie L. Riopel, Lusijah S. Rott and M. Bishr Omary† Palo Alto Veterans Administration Medical Center and the Digestive Disease Center at Stanford University, School of Medicine, 3801 Miranda Avenue, GI 111, Palo Alto, CA 94304, USA *Author for reprint requests †Author for correspondence SUMMARY We studied the solubility of keratin polypeptides 8 and aments in vitro as determined by electron microscopy. 18 (K8/18), which are the predominant intermediate fil- Cross-linking of soluble K8/18 followed by immunopre- aments in the human colonic epithelial cell line HT29. cipitation resulted in dimeric and tetrameric forms, We find that asynchronously growing cells (G0/G1 stage based on migration in SDS-polyacrylamide gels. In of the cell cycle) have a substantial pool of soluble ker- addition, cross-linked and native soluble K8/18 showed atin that constitutes approx. 5% of total cellular ker- similar migration on nondenaturing gels and similar atin. This soluble keratin pool was observed after sedimentation after sucrose density gradient centrifu- immunoprecipitation of K8/18 from the cytosolic frac- gation. Our results indicate that simple epithelial ker- tion of cells disrupted using three detergent-free meth- atins are appreciably more soluble than previously rec- ods. Several other cell lines showed a similar significant ognized. The soluble keratin form is assembly competent soluble cytosolic K8/18 pool. -
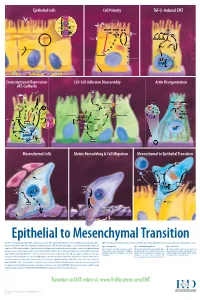
Epithelial to Mesenchymal Transition
Epithelial Cells Cell Polarity TGF-b-Induced EMT MUC-1 O-glycosylation Epithelial Cells ZO-1 Occludin Apical Membrane Tight F-Actin Microvilli Junction Claudin F-Actin p120 β-Catenin Adherens F-Actin Ezrin TGF-β dimer Junction E-Cadherin α-Catenin Plakophilin Crumbs Complex PAR Complex Desmocollin Desmoplakin Desmosome PtdIns(4,5)P2 TGF-β RII TGF-β RI CRB Cdc42Par6 Desmoglein Cytokeratin Pals1 PatJ Tight Junction Plakoglobin aPKC Par3 Domain Smad7 Extracellular PTEN JNK ERK1/2 p38 SARA Smurf1 Cortical Actin Cytoskeleton Space Par3 ZO-1 Adherens Junction PI 3-K Domain Smad-independent Signaling (–) Smad7 Translocation Smad2/3 PtdIns(3,4,5)P3 Smad4 Smad4 NEDD4 Cytokeratin Intermediate Filaments Smad2 Smad4 Smad3 LLGL Proteasome SCRIB DLG Scribble Complex Fibronectin Twist Smad2/3 Vitronectin ZEB 1/2 Microtubule Network Smad4 N-Cadherin Snail Basolateral Membrane CoA, Collagen I Slug CoR MMPs DNA-binding (+) Claudin Desmoplakin Transcription Factor Occludin Cytokeratins E-Cadherin Plakoglobin Integrins β α Nidogen-1/Entactin Perlecan Laminin Collagen IV Transcriptional Repression Cell-Cell Adhesion Disassembly Actin Reorganization of E-Cadherin TGF-β dimer EGF TGF-β RII TGF-β RI IGF FGF Receptor TNF-α Tyrosine Kinase Par6 TNF RI Apical Focal Adhesion Constriction Actin Depolymerization F-Actin Smurf1 Occludin Wnt Frizzled Myosin II Ras RhoA α-Actinin Myosin II ROCK AxinCK1 Dishevelled GSK-3 PI 3-K Src Zyxin MLC Phosphatase APC Proteasome FAK Vinculin RhoA ILK Talin (Inactive) Hakai Talin FAK F-Actin E-Cadherin LIMK Akt Paxillin FAK Stress -

Structures of the ß-Keratin Filaments and Keratin Intermediate Filaments in the Epidermal Appendages of Birds and Reptiles (Sauropsids)
G C A T T A C G G C A T genes Review Structures of the ß-Keratin Filaments and Keratin Intermediate Filaments in the Epidermal Appendages of Birds and Reptiles (Sauropsids) David A.D. Parry School of Fundamental Sciences, Massey University, Private Bag 11-222, Palmerston North 4442, New Zealand; [email protected]; Tel.: +64-6-9517620; Fax: +64-6-3557953 Abstract: The epidermal appendages of birds and reptiles (the sauropsids) include claws, scales, and feathers. Each has specialized physical properties that facilitate movement, thermal insulation, defence mechanisms, and/or the catching of prey. The mechanical attributes of each of these appendages originate from its fibril-matrix texture, where the two filamentous structures present, i.e., the corneous ß-proteins (CBP or ß-keratins) that form 3.4 nm diameter filaments and the α-fibrous molecules that form the 7–10 nm diameter keratin intermediate filaments (KIF), provide much of the required tensile properties. The matrix, which is composed of the terminal domains of the KIF molecules and the proteins of the epidermal differentiation complex (EDC) (and which include the terminal domains of the CBP), provides the appendages, with their ability to resist compression and torsion. Only by knowing the detailed structures of the individual components and the manner in which they interact with one another will a full understanding be gained of the physical properties of the tissues as a whole. Towards that end, newly-derived aspects of the detailed conformations of the two filamentous structures will be discussed and then placed in the context of former knowledge. -

Damage of Hair Follicle Stem Cells and Alteration of Keratin Expression in External Radiation-Induced Acute Alopecia
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 30: 579-584, 2012 Damage of hair follicle stem cells and alteration of keratin expression in external radiation-induced acute alopecia NAOKI NANASHIMA, KOICHI ITO, TAKASHI ISHIKAWA, MANABU NAKANO and TOSHIYA NAKAMURA Department of Biomedical Sciences, Division of Medical Life Sciences, Hirosaki University Graduate School of Health Sciences, Hirosaki, Japan Received April 4, 2012; Accepted May 28, 2012 DOI: 10.3892/ijmm.2012.1018 Abstract. Alopecia is known as a symptom of acute radia- disturbances and blood and bone marrow disorders are known tion, yet little is known concerning the mechanism of this to occur within several hours to several weeks after 1-6 Gy of phenomenon and the alteration of hair protein profiles. To radiation exposure (4,6). examine this, 6-week-old male C57/BL6 mice were exposed Hair loss is also an effect of ARS, but little is known to 6 Gy of X-ray irradiation, which caused acute alopecia. about the mechanism underlying radiation-induced hair loss. Their hair and skin were collected, and hair proteins were In humans, hair loss is caused by radiation of more than analyzed with liquid chromatography/electrospray-ionization 3 Gy, and almost complete hair loss occurs within weeks of mass spectrometry and immunohistochemistry. No change exposure to 6 Gy (4,6). Since blood stem cells are sensitive to was observed in the composition of major hair keratins, such radiation (7), hair loss is thought to be caused by irradiation- as Krt81, Krt83 and Krt86. However, cytokeratin Krt15 and induced stem cell damage, yet no studies have investigated CD34, which are known as hair follicle stem cell markers, this hypothesis. -

A Dysfunctional Desmin Mutation in a Patient with Severe Generalized Myopathy
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 11312–11317, September 1998 Genetics A dysfunctional desmin mutation in a patient with severe generalized myopathy ANA M. MUN˜OZ-MA´RMOL*†‡,GERALDINE STRASSER‡§,MARCOS ISAMAT*†,PIERRE A. COULOMBE¶,YANMIN YANG§, i XAVIER ROCA†,ELENA VELA*†,JOSE´ L. MATE†,JAUME COLL ,MARI´A TERESA FERNA´NDEZ-FIGUERAS†, JOSE´ J. NAVAS-PALACIOS†,AURELIO ARIZA†, AND ELAINE FUCHS§** *Fundacio´n Echevarne, 08037 Barcelona, Spain; Departments of †Pathology and iNeurology, Hospital Universitari Germans Trias i Pujol, Universitat Auto´noma de Barcelona, 08916 Badalona, Barcelona, Spain; ¶Department of Biological Chemistry, The Johns Hopkins University School of Medicine, Baltimore, MD, 21205; and §Howard Hughes Medical Institute and Department of Molecular Genetics and Cell Biology, The University of Chicago, Chicago, IL 60637 Contributed by Elaine Fuchs, July 29, 1998 ABSTRACT Mice lacking desmin produce muscle fibers desmin functions in cellular transmission of both active and with Z disks and normal sarcomeric organization. However, passive force (14, 15). the muscles are mechanically fragile and degenerate upon Desminopathies are a heterogeneous group of human myop- repeated contractions. We report here a human patient with athies characterized by abnormal aggregations of desmin in severe generalized myopathy and aberrant intrasarcoplasmic skeletal andyor cardiac muscle fibers (for review, see ref. 16). The accumulation of desmin intermediate filaments. Muscle tissue clumping of desmin in muscle cells of these patients bears a from this patient lacks the wild-type desmin allele and has a striking resemblance to the keratin aggregates that accumulate in desmin gene mutation encoding a 7-aa deletion within the degenerating epithelial cells of various human keratin disorders coiled-coil segment of the protein. -

Cytoskeletal Remodeling in Cancer
biology Review Cytoskeletal Remodeling in Cancer Jaya Aseervatham Department of Ophthalmology, University of Texas Health Science Center at Houston, Houston, TX 77054, USA; [email protected]; Tel.: +146-9767-0166 Received: 15 October 2020; Accepted: 4 November 2020; Published: 7 November 2020 Simple Summary: Cell migration is an essential process from embryogenesis to cell death. This is tightly regulated by numerous proteins that help in proper functioning of the cell. In diseases like cancer, this process is deregulated and helps in the dissemination of tumor cells from the primary site to secondary sites initiating the process of metastasis. For metastasis to be efficient, cytoskeletal components like actin, myosin, and intermediate filaments and their associated proteins should co-ordinate in an orderly fashion leading to the formation of many cellular protrusions-like lamellipodia and filopodia and invadopodia. Knowledge of this process is the key to control metastasis of cancer cells that leads to death in 90% of the patients. The focus of this review is giving an overall understanding of these process, concentrating on the changes in protein association and regulation and how the tumor cells use it to their advantage. Since the expression of cytoskeletal proteins can be directly related to the degree of malignancy, knowledge about these proteins will provide powerful tools to improve both cancer prognosis and treatment. Abstract: Successful metastasis depends on cell invasion, migration, host immune escape, extravasation, and angiogenesis. The process of cell invasion and migration relies on the dynamic changes taking place in the cytoskeletal components; actin, tubulin and intermediate filaments. This is possible due to the plasticity of the cytoskeleton and coordinated action of all the three, is crucial for the process of metastasis from the primary site. -

Keratins Determine Network Stress Responsiveness in Reconstituted Actin-Keratin Filament Systems
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.27.424392; this version posted December 28, 2020. The copyright holder for this preprint (which was not certified by peer review)Please is the author/funder, do not adjust who margins has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. ARTICLE Keratins determine network stress responsiveness in reconstituted actin‐keratin filament systems† *af‡ ab‡ ab cd *abe Iman Elbalasy , Paul Mollenkopf , Cary Tutmarc , Harald Herrmann and Jörg Schnauß The cytoskeleton is a major determinant of cell mechanics, a property that is altered during many pathological situations. To understand these alterations, it is essential to investigate the interplay between the main filament systems of the cytoskeleton in the form of composite networks. Here, we investigate the role of keratin intermediate filaments (IFs) in network strength by studying in vitro reconstituted actin and keratin 8/18 composite networks via bulk shear rheology. We co‐polymerized these structural proteins in varying ratios and recorded how their relative content affects the overall mechanical response of the various composites. For relatively small deformations, we found that all composites exhibited an intermediate linear viscoelastic behavior compared to that of the pure networks. In stark contrast, the composites displayed increasing strain stiffening behavior as a result of increased keratin content when larger deformations were imposed. This strain stiffening behavior is fundamentally different from behavior encountered with vimentin IF as a composite network partner for actin. Our results provide new insights into the mechanical interplay between actin and keratin in which keratin provides reinforcement to actin. -

Biological Functions of Cytokeratin 18 in Cancer
Published OnlineFirst March 27, 2012; DOI: 10.1158/1541-7786.MCR-11-0222 Molecular Cancer Review Research Biological Functions of Cytokeratin 18 in Cancer Yu-Rong Weng1,2, Yun Cui1,2, and Jing-Yuan Fang1,2,3 Abstract The structural proteins cytokeratin 18 (CK18) and its coexpressed complementary partner CK8 are expressed in a variety of adult epithelial organs and may play a role in carcinogenesis. In this study, we focused on the biological functions of CK18, which is thought to modulate intracellular signaling and operates in conjunction with various related proteins. CK18 may affect carcinogenesis through several signaling pathways, including the phosphoinosi- tide 3-kinase (PI3K)/Akt, Wnt, and extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) signaling pathways. CK18 acts as an identical target of Akt in the PI3K/Akt pathway and of ERK1/2 in the ERK MAPK pathway, and regulation of CK18 by Wnt is involved in Akt activation. Finally, we discuss the importance of gaining a more complete understanding of the expression of CK18 during carcinogenesis, and suggest potential clinical applications of that understanding. Mol Cancer Res; 10(4); 1–9. Ó2012 AACR. Introduction epithelial organs, such as the liver, lung, kidney, pancreas, The intermediate filaments consist of a large number of gastrointestinal tract, and mammary gland, and are also nuclear and cytoplasmic proteins that are expressed in a expressed by cancers that arise from these tissues (7). In the tissue- and differentiation-dependent manner. The compo- absence of CK8, the CK18 protein is degraded and keratin fi intermediate filaments are not formed (8). -

Cutaneous Dermoid Cyst: Cytokeratin and Filaggrin Expression Suggesting Differentiation Towards Follicular Infundibulum and Mature Sebaceous Gland
295-299 3/7/06 18:45 Page 295 ONCOLOGY REPORTS 16: 295-299, 2006 295 Cutaneous dermoid cyst: Cytokeratin and filaggrin expression suggesting differentiation towards follicular infundibulum and mature sebaceous gland ICHIRO KUROKAWA1, KEISUKE NISHIMURA1, ARATA HAKAMADA1, KEN-ICHI ISODA1, KEI-ICHI YAMANAKA1, HITOSHI MIZUTANI1 and AIRO TSUBURA2 1Department of Dermatology, Mie University Graduate School of Medicine, Tsu, Mie 514-8507; 2Department of Pathology, Kansai Medical University, Moriguchi, Osaka 570-8506, Japan Received February 20, 2006; Accepted April 10, 2006 Abstract. We experienced two cases of cutaneous dermoid To elucidate the histogenesis of DC, we studied CK and cysts (DC). To elucidate the histogenesis of DC, we have filaggrin expression in DC using ten different anti-keratin studied cytokeratin (CK) expression in DC using ten different antibodies against CK1, 7, 8, 10, 14, 15, 16, 17, 18 and 19, anti-keratin antibodies against CK1, 7, 8, 10, 14, 15, 16, 17, and anti-filaggrin antibody. 18 and 19, and anti-filaggrin (filament aggregating protein) antibody. In the cyst wall of DC, CK1 and 10 were expressed Materials and methods in suprabasal layer, and CK14 was limited to the basal layer. In sebaceous gland-like structures, CK14 was detected in The patient, a 48-year-old male had DC located on the left sebaceous acinus, and CK17 was detected in sebaceous duct. postauriclar area from at birth. The other patient, a 31-year- The other CKs were not detected. Filaggrin was intensely old female developed DC on the right postauriclar area ten detected in the granular layer in the cyst wall of DC. -

Deciphering the Mechanoresponsive Role of Β-Catenin in Keratoconus
bioRxiv preprint doi: https://doi.org/10.1101/603738; this version posted April 9, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Deciphering the mechanoresponsive role of β-catenin in Keratoconus 2 epithelium 3 4 Chatterjee Amit 1,2, Prema Padmanabhan3, Janakiraman Narayanan#1 5 1 Department of Nanobiotechnology, Vision Research Foundation,Sankara Nethralaya campus, 6 Chennai, Tamil Nadu, India 7 2 School of Chemical and Biotechnology, SASTRA University, Tanjore, Tamil Nadu, India 8 3 Department of Cornea, Medical Research Foundation, Sankara Nethralaya campus, Chennai, 9 Tamil Nadu ,India 10 Running Title: β-catenin mechanotransduction in kerataconus 11 Correspondence: #Dr. Janakiraman Narayanan, Department of Nanobiotechnology, KNBIRVO 12 Block, Vision Research Foundation, Sankara Nethralaya campus 18/41, College road 13 Nungambakkam, Chennai, Tamil Nadu, India 600006, Tel-+91-44-28271616, (Ext) 1358, Fax- 14 +91-44-28254180, Email: [email protected] 15 16 17 18 19 20 21 22 23 1 bioRxiv preprint doi: https://doi.org/10.1101/603738; this version posted April 9, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 24 25 Abstract 26 Keratoconus (KC) a disease with established biomechanical instability of the corneal stroma, is an 27 ideal platform to identify key proteins involved in mechanosensing.