Specificity, Rank Preference, and the Colonization of a Non-Native Host
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

C10 Beano2.Gen-Wis
LEGUMINOSAE PART DEUX Papilionoideae, Genista to Wisteria Revised May the 4th 2015 BEAN FAMILY 2 Pediomelum PAPILIONACEAE cont. Genista Petalostemum Glycine Pisum Glycyrrhiza Psoralea Hylodesmum Psoralidium Lathyrus Robinia Lespedeza Securigera Lotus Strophostyles Lupinus Tephrosia Medicago Thermopsis Melilotus Trifolium Onobrychis Vicia Orbexilum Wisteria Oxytropis Copyrighted Draft GENISTA Linnaeus DYER’S GREENWEED Fabaceae Genista Genis'ta (jen-IS-ta or gen-IS-ta) from a Latin name, the Plantagenet kings & queens of England took their name, planta genesta, from story of William the Conqueror, as setting sail for England, plucked a plant holding tenaciously to a rock on the shore, stuck it in his helmet as symbol to hold fast in risky undertaking; from Latin genista (genesta) -ae f, the plant broom. Alternately from Celtic gen, or French genet, a small shrub (w73). A genus of 80-90 spp of small trees, shrubs, & herbs native of Eurasia. Genista tinctoria Linnaeus 1753 DYER’S GREENWEED, aka DYER’S BROOM, WOADWAXEN, WOODWAXEN, (tinctorius -a -um tinctor'ius (tink-TORE-ee-us or tink-TO-ree-us) New Latin, of or pertaining to dyes or able to dye, used in dyes or in dyeing, from Latin tingo, tingere, tinxi, tinctus, to wet, to soak in color; to dye, & -orius, capability, functionality, or resulting action, as in tincture; alternately Latin tinctōrius used by Pliny, from tinctōrem, dyer; at times, referring to a plant that exudes some kind of stain when broken.) An escaped shrub introduced from Europe. Shrubby, from long, woody roots. The whole plant dyes yellow, & when mixed with Woad, green. Blooms August. Now, where did I put that woad? Sow at 18-22ºC (64-71ºF) for 2-4 wks, move to -4 to +4ºC (34-39ºF) for 4-6 wks, move to 5-12ºC (41- 53ºF) for germination (tchn). -

Butterflies of Kootenai County 958 South Lochsa St Post Falls, ID 83854
Butterflies of Kootenai County 958 South Lochsa St Post Falls, ID 83854 Phone: (208) 292-2525 Adapted from Oregon State University Extension FAX: (208) 292-2670 Booklet EC 1549 and compiled by Mary V., Certified E-mail: [email protected] Idaho Master Gardener. Web: uidaho.edu/kootenai By growing a bounty of native plants, mixed with nearly-natives or non-natives, you can attract a variety of butterflies. Additional reading: https://xerces.org/your-pollinator-garden/ Butterflies favor platform-shaped flowers but will feed on a diversity of nectar-rich http://millionpollinatorgardens.org/ flowers. They prefer purple, red, orange, https://www.fs.fed.us/wildflowers/pollinator violet, and yellow flower colors with sweet s/documents/AttractingPollinatorsV5.pdf scents. Butterflies love warm, sunny and http://xerces.org/pollinators-mountain- windless weather. region/ Planning your garden – Think like a o Tolerate Damage on your Plants: A butterfly Pollinator garden needs plants that feed larvae o Go Native: Pollinators are best adapted to (caterpillars). They feed on leaves and plant local, native plants which often need less material. If you do not feed the young, the water than ornamentals. adults will not stay in your landscapes. o Plant in Groups of three or more: Planting o Provide a puddle as a water source: Allow large patches of each plant species for better water to puddle in a rock or provide a foraging efficiency. shallow dish filled with sand as a water source for butterflies. Float corks or a stick o Blooming All Season: Flowers should bloom in your garden throughout the in the puddles to allow insects that fall in to growing season. -

Karner Blue Butterfly (Lycaeides Melissa Samuelis) 5-Year Review
FINAL Karner Blue Butterfly (Lycaeides melissa samuelis) 5-Year Review: Summary and Evaluation U.S. Fish and Wildlife Service Ecological Services Field Office New Franken, Wisconsin 2012 TABLE OF CONTENTS 1. GENERAL INFORMATION 1.1 Reviewers……………………………………………………………………………. 1 1.2 Methodology used to complete the review …………………………………………. 1 1.3 Background …………………………………………………………………………. 1 2. REVIEW ANALYSIS 2.1 Application of the 1996 Distinct Population Segment (DPS) policy……………….. 2 2.2 Recovery Criteria…………………………………………………………………… 2 2.3 Updated Information and Current Species Status…………………………………..10 2.3.1 Biology and Habitat………………………………………………………….. 10 2.3.1.1 New information on the species’ biology and life history…………….10 KBB Eggs………………………………………………………….... 10 Larval and Pupal Growth……………………………………………..10 Larval Behavior………………………………………………………10 Oviposition Behavior and Patterns…………………………………...11 Effect of Habitat Management on Oviposition…………………….…12 Ovipositioning Rates of Wild and Captive Bred KBBs…………...…12 Effect of Subhabitat on Adult Production……………………………12 Wild Lupine (Host Plant)……………………………………………. 13 Nectar Plants and Adult Foraging Behavior………………………… 18 Habitat Characteristics Considered for Reintroductions in Ontario……………………………………………………… 21 Dispersal…………………………………………………………….. 22 KBB – a Flagship Species………………………………………..…...23 2.3.1.2 Abundance, population trends, demographic features or trends……. 23 Lifespan……………………………………………………………… 23 KBB Metapopulation Dynamics and Population Growth Rates…….. 24 Brood Size, Brood Number and Growth Rates……………………… -

Karner Blue (Lycaeides Melissa Samuelis) Butterfly in the USA (After Seal 1992)
COSEWIC Assessment and Update Status Report on the Karner Blue Lycaeides melissa samuelis in Canada EXTIRPATED 2000 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: Please note: Persons wishing to cite data in the report should refer to the report (and cite the author(s)); persons wishing to cite the COSEWIC status will refer to the assessment (and cite COSEWIC). A production note will be provided if additional information on the status report history is required. COSEWIC. 2000. COSEWIC assessment and update status report on the Karner Blue Lycaeides melissa samuelis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. v + 20 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Previous report(s): Carson J.P. 1997. COSEWIC status report on the Karner Blue Butterfly Lycaeides melissa samuelis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1 - 22 pp. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Ếgalement disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la situation sur le bleu mélissa (Lycaeides melissa samuelis) au Canada – Mise à jour. Cover illustration: Karner Blue — Illustration by Peter Burke. ©Her Majesty the Queen in Right of Canada, 2010. Catalogue No. CW69-14/131-2000E-PDF ISBN 978-1-100-16452-6 Recycled paper COSEWIC Assessment Summary Assessment summary – November 2000 Common name Karner Blue Scientific name Lycaeides melissa samuelis Status Extirpated Reason for designation This species and its habitat originally occurred in a restricted range. -

Northern Blue — Wisconsinbutterflies.Org Page 1 of 6
Northern Blue — wisconsinbutterflies.org Page 1 of 6 Wisconsin Butterflies z butterflies z tiger beetles z robber flies Search species Northern Blue Lycaeides idas The Northern Blue has been found only in the far northeastern counties in Wisconsin. The ‘Karner’ Melissa Blue is a very similar species that was not listed in the 1970 book, “Butterflies of Wisconsin” by Ebner. Several records of the Northern Blue that were referred to in that book were probably of that species. The larvae host plant of the Northern Blue is Dwarf Bilberry (Vaccinium caespitosum), while that of the Karner Blue is Wild Lupine (Lupinus perennis). The habitats and general distributions of these plants are very different and it is unlikely that both would be found in the same area. Weekly sightings for Northern Blue Identifying characteristics Above, the male is a light blue with a thin black margin and a light fringe. The female is a black towards the margins with varying amounts of blue on both wings and submarginal orange spots that may even be absent in some individuals. Mo Nielsen in his “Michigan Butterflies & Skippers” has a photo of a female that has no orange spots above. Underneath the sexes are essentially the same, a light gray background, very prominent black spots, and a row of silvery spots topped by orange and then black crescents. Notice that extremely worn individuals, like the bottom one with no fringe left at all, may have no obvious orange spots below. Similar species In Wisconsin this species could be confused with the ‘Karner’ Melissa Blue, but the ranges of these species do not overlap and they are not found in the same habitat. -

Butterfly Plants
VisitWimberley.com List of Plants, with Butterfly and Caterpillar Feeding Information Page 1 Plant Name Scientific Plant These butterflies feed on These caterpillars feed on Name the plants nectar… the plant… Callichamys Statira Sulphur latifolia Desmodium Common Longtail, Dorantes tortuosum Longtail, Gray Hairstreak (Strymon melinus), Cassius Blue, Tailed Blue, Variegated Fritillary (Euptoieta claudia), Tailed Orange Dicliptera Texan Crescent brachiator E. betonicifolium Lost Metalmark Eupatorium Rawson's Metalmark greggii Lantana spp x Lomatium Pergamus Swallowtail lucidum (Papilio indra pergamus) Loosestrife spp. x P. adenopoda, P. Sierran Fritillary capsularis P. affinis Gulf Fritillary (Agraulis vanillae), Zebra P. platyloba Flambeau, Small Tiger Physostegia x virginiana Ruellia Texan Crescent carolinensis Ruellia Common Buckeye, occidentalis Mexican Buckeye, White Peacock Stemodia Black Buckeye tomentosa Tauschia arguta Pergamus Swallowtail (Papilio indra pergamus) Tetrastylis lobata Sierran Fritillary, Zebra Turnera Variegated Fritillary (Euptoieta claudia), Mexican fritillary Umbelliferae Pergamus Swallowtail (Papilio indra pergamus) Weigela spp. x Abelia, Glossy Abelia grandiflora x Abrojo Lacinia Patch Achillea Achillea x Millefolium Agarita Berberis trifoliata x Ageratum Ageratum x houstonianum Alfalfa Medicago sativa Parsnip or Black Swallowtail Clouded Sulphur (Colias (Papilio polyxenes asterius), philodice philodice), Melissa Checkered White (Pontia Blue protodice), Clouded Sulphur (Lycaeides melissa), Orange (Colias philodice -
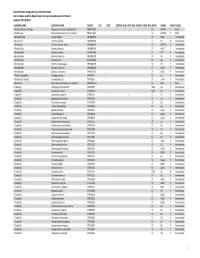
Element Status Designations by Common Name Arizona Game And
Element Status Designations by Common Name Arizona Game and Fish Department, Heritage Data Management System Updated: 10/15/2019 COMMON NAME SCIENTIFIC NAME ELCODE ESA DATE CRITHAB BLM USFS NESL MEXFED SGCN NPL SRANK GRANK TRACK TAXON A Arizona‐Mexican Orange Choisya arizonica var. amplophylla PDRUT02031 S2 G4TNR Y Plant A Balsamroot Balsamorhiza hookeri var. hispidula PDAST11041 S1 G5T3T5 Y Plant A Blueberry Bee Osmia ribifloris IIHYMA2570 S? G4G5 Y Invertebrate A Buckmoth Hemileuca grotei IILEW0M070 S? G4 N Invertebrate A Buckmoth Hemileuca grotei diana IILEW0M072 S? G4T3T4 Y Invertebrate A Bumble Bee Bombus centralis IIHYM24100 S? G4G5 Y Invertebrate A Bumble Bee Bombus fervidus IIHYM24110 S? G4? Y Invertebrate A Bumble Bee Bombus flavifrons IIHYM24120 S? G5 Y Invertebrate A Bumble Bee Bombus huntii IIHYM24140 S? G5 Y Invertebrate A Bumble Bee Bombus melanopygus IIHYM24150 S? G5 Y Invertebrate A Bumble Bee Bombus morrisoni IIHYM24460 S? G4G5 Y Invertebrate A Bumble Bee Bombus nevadensis IIHYM24170 S? G4G5 Y Invertebrate A Bushtail Caddisfly Gumaga griseola IITRI53010 S? G5 Y Invertebrate A Bushtailed Caddisfly Gumaga nigricula IITRI53020 S? G3G4 Y Invertebrate A Buttercup Ranunculus inamoenus var. subaffinis PDRAN0L1C3 S1 G5T1 YPlant A caddisfly Hydropsyche occidentalis IITRI25460 S2S3 G5 Y Invertebrate A caddisfly Hydropsyche oslari IITRIG6010 S2S3 G5 Y Invertebrate A caddisfly Lepidostoma apache IITRI64A10 S S1 G1 Y Invertebrate A Caddisfly Agapetus boulderensis IITRI33190 S? G5 Y Invertebrate A Caddisfly Alisotrichia arizonica IITRID7010 -

Papilio Series) 2006
(NEW April 28 PAPILIO SERIES) 2006 TAXONOMIC STUDIES AND NEW TAXA OF NORTH AMERICAN BUTTERFLIES by James A. Scott (also editor), Michael S. Fisher, Norbert G. Kondla, Steve Kohler, Crispin S. Guppy, Stephen M. Spomer, and B. Chris Schmidt Abstract. New diversity is reported and discussed among North American butterflies. Several dozen new taxa are named. A new "sibling" species has been found to occur throughout the Rocky Mts., introducing a new butterfly species to most states in western U.S. and to southern Alberta and BC. Several taxa of Colias, Euphydryas, Lycaena, and Plebejus are raised to species status. Many nam.e changes are made, and many taxa are switched between species to create several dozen new combinations. The relevance of species concepts to difficult groups of butterflies is explored. Introduction This paper consists of miscellaneous taxonomic studies on North American butterflies, some in the northeast, but mostly in the west. Most of the diversity of butterfly fauna in North America is in the western mountainous areas, where the human population is lower, so it has taken longer to study western butterflies, and a lot more study is needed. We have made new findings on many wes.tern butterflies, and this progress is reported below. And Scott recently moved his collection out of old dermestid-infested drawers into fine very-tight ones that those beetles cannot enter, and in the process of resorting them found a dozen unnamed subspecies, which are named below. As we study our butterflies and learn more and more about them, a disturbing pattern has emerged. -

Population Biology of an Endangered Butterfly, Lycaeides Melissa Samuelis (Lepidoptera; Lycaenidae): Genetic Variation, Gene Flow, and Taxonomic Status
320 Population biology of an endangered butterfly, Lycaeides melissa samuelis (Lepidoptera; Lycaenidae): genetic variation, gene flow, and taxonomic status Laurence Packer, John S. Taylor, Dolores A. Savignano, Catherine A. Bleser, Cynthia P. Lane, and Laura A. Sommers Abstract: We present data from 34 allozyme loci to test whether the Karner Blue butterfly is specifically differentiated from the Melissa Blue. Furthermore, as the Karner Blue is an endangered organism of low vagility that occurs predominantly in small, widely separated populations, we investigated (i) whether the Karner Blue is depauperate in genetic variation and (ii) whether gene flow between sampled populations is unusually low. Genetic identities between New York and Wisconsin populations of the Karner Blue and a sample of Melissa Blue from Minnesota are all statistically indistinguishable. Neither genetic identity data nor application of the phylogenetic species concept support formal recognition of the Karner Blue as a species separate from the Melissa Blue. Nonetheless, the data indicate that gene flow among the samples was very low compared with that among populations of other Lepidoptera. Heterozygosity estimates for all three samples were comparable to data for other Lepidoptera and indicate that the Karner Blue populations surveyed are not under immediate threat of extirpation due to loss of genetic diversity. Although the available data are limited, if the Karner Blue is to be managed as an evolutionarily significant unit, then the eastern and western populations should probably be treated independently and each should receive high conservation priority. Résumé : Nous présentons ici les résultats d’une analyse des allozymes à 34 locus entreprise dans le but de déterminer si le Bleu de Karner est une espèce distincte du Bleu melissa. -

Ecological Sustainability Analysis of the Kaibab National Forest
Ecological Sustainability Analysis of the Kaibab National Forest: Species Diversity Report Version 1.2.5 Including edits responding to comments on version 1.2 Prepared by: Mikele Painter and Valerie Stein Foster Kaibab National Forest For: Kaibab National Forest Plan Revision Analysis 29 June 2008 SDR version 1.2.5 29 June 2008 Table of Contents Table of Contents ............................................................................................................................. i Introduction ..................................................................................................................................... 1 PART I: Species Diversity .............................................................................................................. 1 Species Diversity Database and Forest Planning Species........................................................... 1 Criteria .................................................................................................................................... 2 Assessment Sources ................................................................................................................ 3 Screening Results .................................................................................................................... 4 Habitat Associations and Initial Species Groups ........................................................................ 8 Species associated with ecosystem diversity characteristics of terrestrial vegetation or aquatic systems ...................................................................................................................... -

Karner Blue (Lycaeides Melissa Samuelis), Frosted Elfin (Callophrys Irus), and Eastern Persius Duskywing (Erynnis Persius Persius) in Canada
Species at Risk Act Recovery Strategy Series Recovery Strategy for the Karner Blue (Lycaeides melissa samuelis), Frosted Elfin (Callophrys irus), and Eastern Persius Duskywing (Erynnis persius persius) in Canada Karner Blue Frosted Elfin Eastern Persius Duskywing Karner Blue Eastern Persius Duskywing Frosted Elfin 2019 Recommended citation: Environment and Climate Change Canada. 2019. Recovery Strategy for the Karner Blue (Lycaeides melissa samuelis), Frosted Elfin (Callophrys irus) and Eastern Persius Duskywing (Erynnis persius persius) in Canada. Species at Risk Act Recovery Strategy Series. Environment and Climate Change Canada, Ottawa. xv + 71 pp. For copies of the recovery strategy, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry1. Cover photos: Left: Karner Blue photo taken by Bob Yukich, Allegan State Game Area, Michigan; Center: Frosted Elfin photo taken by Ann B. Swengel, Jackson, Wisconsin; Right: Male Eastern Persius Duskywing showing dorsal (left) and ventral (right) views (specimen plate prepared by John Fowler). Également disponible en français sous le titre « Programme de rétablissement du bleu mélissa (Lycaeides melissa samuelis), du lutin givré (Callophrys irus) et de l’hespérie Persius de l’Est (Erynnis persius persius) au Canada » © Her Majesty the Queen in Right of Canada, represented by the Minister of Environment and Climate Change, 2019. All rights reserved. ISBN 978-0-660-28326-5 Catalogue no. En3-4/299-2019E-PDF Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. -

Inventories of Butterflies 2009
2009 Report Inventories of Butterflies in Boulder County By Janet Chu November 7, 2009 Table of Contents I. Acknowledgments …………………… 3 II. Abstract …………………………… 4 II. Introduction……………………………… 4 IV. Objectives ………………………….. 5 V. Research Methods ………………….. 6 VI. Results and Discussion ………………... 6 A. Boulder County Open Spaces ... 7 B. City of Boulder Properties …. 9 VII. Conclusions …………………………….. 13 VIII. Recommendations …………………….. 13 IX. References …………………………. 14 X. Butterfly Survey Data Tables …………. 15 Table I. Survey Dates and Locations ……………. 15 Sites belonging to Boulder County … 15 Table II. Southeast Buffer …………………. 16 Table III. Anne U. White Trail ………………. 18 Table IV. Heil Valley Open Space –Geer Watershed... 21 Table V. Heil Valley Open Space –Plumely Canyon 24 Table VI. Heil Valley Open Space – North ………… 27 Table VII. Walker Ranch - Meyer‟s Gulch ………… 30 Table VIII. Caribou Ranch Open Space ………………33 Sites belonging to City of Boulder …… 36 Table IX. Left Hand Valley Reservoir …………… 36 Table X. Hoover Hill, Westview Road ……………. 37 Table XI. Marshall Mesa …………………….…… 38 2 I. Acknowledgments Our research team has conducted butterfly surveys for eight consecutive years, from 2002 through 2009, with 2002-2004 being introductory to the lands and species, and 2005-2009 more in depth. Larry Crowley, Jean Morgan, and Amy Chu have been valuable team members, joined at times by the author‟s grandsons Asa and Jeremy Hurst and associates Mike Sportiello and Cathy Cook. Ruth Carol Cushman and Joyce Gellhorn added expertise on plant life. The majority of the surveys have been in Boulder County Parks and Open Space (BCPOS) lands. Therese Glowacki issued a Special Collection Permit for access into the Open Spaces; Mark Brennan oversaw research, maintained records of our monographs and organized seminars for presentation of data.