Transcript Quantification and Gene-Fusion Detection Using Long Reads
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Université De La Méditerranée Faculte De Médecine De Marseille École Doctorale Des Sciences De La Vie Et De La Santé Centre De Recherche En Cancérologie De Marseille
UNIVERSITÉ DE LA MÉDITERRANÉE FACULTE DE MÉDECINE DE MARSEILLE ÉCOLE DOCTORALE DES SCIENCES DE LA VIE ET DE LA SANTÉ CENTRE DE RECHERCHE EN CANCÉROLOGIE DE MARSEILLE T H È S E Pour l’obtention du Diplôme de DOCTEUR de L’UNIVERSITÉ de la MÉDITERRANÉE SPÉCIALITÉ : Oncologie : Pharmacologie et Thérapeutique Présentée et soutenue publiquement par Mme Laetitia STUHL-GOURMAND Le 30 Mars 2010 ROLE DE NACA ET DE SES PARTENAIRES MOLÉCULAIRES DANS LA DIFFÉRENCIATION ÉRYTHROÏDE NORMALE ET PATHOLOGIQUE DES SYNDROMES MYÉLODYSPLASIQUES Directeur de Thèse : Dr Sophie GOMEZ Membres du Jury de Thèse : Pr Daniel Olive Président Pr Catherine Lacombe Rapporteur Dr Dominique Duménil Rapporteur Dr Christian Chabannon Examinateur 1 A mes deux hommes, mes deux amours : mon fils Anthony et mon mari Sébastien 2 REMERCIEMENTS Je remercie les membres du jury d’avoir accepté de juger ce travail: les Pr Catherine Lacombe, Dr Christian Chabannon, Dr Dominique Duménil et le Pr Daniel Olive de présider ce jury. Je remercie Françoise Birg de m’avoir accueillie au centre de Recherche en Cancérologie de Marseille UMR891. Je remercie ma directrice de thèse Dr Sophie Gomez a qui je dois beaucoup. Merci de m’avoir toujours soutenue tout au long de cette thèse, et de m’avoir accompagné avec patience dans l’aboutissement de ce travail à mon rythme de jeune maman. Je remercie le Dr Patrice Dubreuil de m’avoir accueilli dans son équipe, d’avoir toujours été à l’écoute, et de m’avoir soutenu pour la finalisation de mon projet. Je remercie Véronique Gelsi-Boyer, Virginie Trouplin, Daniel Birnbaum et Norvert Vey, sans qui le projet MDS n’aurait pas existé. -

Naca Mouse Shrna Plasmid (Locus ID 17938) – TL501429 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TL501429 Naca Mouse shRNA Plasmid (Locus ID 17938) Product data: Product Type: shRNA Plasmids Product Name: Naca Mouse shRNA Plasmid (Locus ID 17938) Locus ID: 17938 Synonyms: AL022831; AL024382; Gm1878; mKIAA0363; skNAC Vector: pGFP-C-shLenti (TR30023) Format: Lentiviral plasmids Components: Naca - Mouse, 4 unique 29mer shRNA constructs in lentiviral GFP vector(Gene ID = 17938). 5µg purified plasmid DNA per construct Non-effective 29-mer scrambled shRNA cassette in pGFP-C-shLenti Vector, TR30021, included for free. RefSeq: BC083340, BC099375, NM_001113199, NM_001282976, NM_013608, BC029830 Summary: Prevents inappropriate targeting of non-secretory polypeptides to the endoplasmic reticulum (ER). Binds to nascent polypeptide chains as they emerge from the ribosome and blocks their interaction with the signal recognition particle (SRP), which normally targets nascent secretory peptides to the ER. Also reduces the inherent affinity of ribosomes for protein translocation sites in the ER membrane (M sites) (By similarity). Isoform 1 and isoform 2 appear to bind DNA and play roles in transcription. Isoform 1 may function as a specific coactivator for JUN, acting to stabilize the interaction of JUN homodimers with promoter elements. [UniProtKB/Swiss-Prot Function] shRNA Design: These shRNA constructs were designed against multiple splice variants at this gene locus. To be certain that your variant of interest is targeted, please contact [email protected]. If you need a special design or shRNA sequence, please utilize our custom shRNA service. -

Crystal Structures of NAC Domains of Human Nascent Polypeptide-Associated Complex (NAC) and Its Αnac Subunit
Protein Cell 2010, 1(4): 406–416 Protein & Cell DOI 10.1007/s13238-010-0049-3 RESEARCH ARTICLE Crystal structures of NAC domains of human nascent polypeptide-associated complex (NAC) and its αNAC subunit ✉ Lanfeng Wang1, Wenchi Zhang1, Lu Wang1, Xuejun C.Zhang1, Xuemei Li1, Zihe Rao1,2,3 1 National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, 15 Datun Road, Beijing 100101, China 2 Structure Biology Laboratory, Tsinghua University, Beijing 100084, China 3 Tianjin Key Laboratory of Protein Science, College of Life Sciences, Nankai University, Tianjin 300071, China ✉ Correspondence: [email protected] Received March 28, 2010 Accepted April 12, 2010 ABSTRACT binding with other cytosolic factors (Wiedmann et al., 1994). This complex is widely conserved from archaea to human. Nascent polypeptide associated complex (NAC) and its Some NAC mutants induce early embryonically lethal two isolated subunits, αNAC and βNAC, play important phenotypes in mice, fruit fly, and Caenorhabditis elegans roles in nascent peptide targeting. We determined a 1.9 Å (Deng and Behringer, 1995; Markesich et al., 2000). Experi- resolution crystal structure of the interaction core of NAC ments to determine NAC intracellular localization and heterodimer and a 2.4 Å resolution crystal structure of distribution show that the vast majority of NAC is in the αNAC NAC domain homodimer. These structures provide cytoplasm as a stable heterodimer, and no single subunit and detailed information of NAC heterodimerization and homo-oligomer has been observed under physiologic condi- αNAC homodimerization. We found that the NAC tions (Beatrix et al., 2000). However, drastic variations of domains of αNAC and βNAC share very similar folding relative concentration between the two NAC subunits are despite of their relative low identity of amino acid found in patients of Alzheimer’s disease, Down syndrome, sequences. -

Discovery of RSV-Induced BRD4 Protein Interactions Using Native Immunoprecipitation and Parallel Accumulation – Serial Fragmentation (PASEF) Mass Spectrometry
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 26 February 2021 Article Discovery of RSV-induced BRD4 protein interactions using native immunoprecipitation and parallel accumulation – serial fragmentation (PASEF) mass spectrometry Morgan Mann 1 , David Roberts 2 , Yanlong Zhu 3,4 , Yi Li 5, Jia Zhou 5 , Ying Ge 2,3,4 and Allan R. Brasier 6* 1 Department of Medicine, Division of Endocrinology, Diabetes, and Metabolism, University of Wisconsin-Madison, Madison, WI, 53705, USA 2 Department of Chemistry, University of Wisconsin-Madison, Madison, WI, 53706, USA 3 Human Proteomics Program, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, 53705, USA 4 Department of Cell and Regenerative Biology, University of Wisconsin-Madison, Madison, WI, 53705, USA 5 Department of Pharmacology and Toxicology, University of Texas Medical Branch, Galveston, TX 77550 6 Institute for Clinical and Translational Research (ICTR), University of Wisconsin-Madison, Madison, WI, 53705, USA * Correspondence: [email protected]; Tel.: 1-608-263-7371 1 Abstract: Respiratory Syncytial Virus (RSV) causes severe inflammation and airway pathology 2 in children and the elderly by infecting the epithelial cells of the upper and lower respiratory 3 tract. RSV replication is sensed by intracellular pattern recognition receptors upstream of the IRF 4 and NF-κB transcription factors. These proteins coordinate an innate inflammatory response via 5 Bromodomain containing protein 4 (BRD4), a protein that functions as a scaffold for unknown 6 transcriptional regulators. To better understand the pleiotropic regulatory function of BRD4, 7 we examine the BRD4 interactome and identify how RSV infection dynamically alters it. To 8 accomplish these goals, we leverage native immunoprecipitation and Parallel Accumulation – 9 Serial Fragmentation (PASEF) mass spectrometry to examine BRD4 complexes isolated from 10 human alveolar epithelial cells in the absence or presence of RSV infection. -
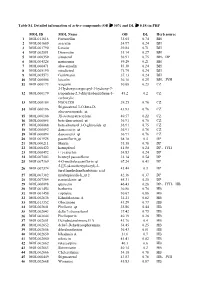
MOL ID MOL Name OB DL Herb Source 1 MOL011616 Fortunellin
Table S1. Detailed information of active compounds (OB ≥ 30% and DL ≥ 0.18) in PHF MOL ID MOL Name OB DL Herb source 1 MOL011616 Fortunellin 35.65 0.74 BH 2 MOL001689 acacetin 34.97 0.24 BH 3 MOL001790 Linarin 39.84 0.71 BH 4 MOL002881 Diosmetin 31.14 0.27 BH 5 MOL000359 sitosterol 36.91 0.75 BH、DP 6 MOL004328 naringenin 59.29 0.21 BH 7 MOL000471 aloe-emodin 83.38 0.24 BH 8 MOL005190 eriodictyol 71.79 0.24 BH 9 MOL005573 Genkwanin 37.13 0.24 BH 10 MOL000006 luteolin 36.16 0.25 BH、JYH 11 MOL000173 wogonin 30.68 0.23 CZ 2-Hydroxyisoxypropyl-3-hydroxy-7- 12 MOL000179 isopentene-2,3-dihydrobenzofuran-5- 45.2 0.2 CZ carboxylic 13 MOL000184 NSC63551 39.25 0.76 CZ Stigmasterol 3-O-beta-D- 14 MOL000186 43.83 0.76 CZ glucopyranoside_qt 15 MOL000188 3β-acetoxyatractylone 40.57 0.22 CZ 16 MOL000085 beta-daucosterol_qt 36.91 0.75 CZ 17 MOL000088 beta-sitosterol 3-O-glucoside_qt 36.91 0.75 CZ 18 MOL000092 daucosterin_qt 36.91 0.76 CZ 19 MOL000094 daucosterol_qt 36.91 0.76 CZ 20 MOL001925 paeoniflorin_qt 68.18 0.4 DP 21 MOL000211 Mairin 55.38 0.78 DP 22 MOL000422 kaempferol 41.88 0.24 DP、JYH 23 MOL000492 (+)-catechin 54.83 0.24 DP 24 MOL007003 benzoyl paeoniflorin 31.14 0.54 DP 25 MOL007369 4-O-methylpaeoniflorin_qt 67.24 0.43 DP 5-[[5-(4-methoxyphenyl)-2- 26 MOL007374 43.44 0.3 DP furyl]methylene]barbituric acid 27 MOL007382 mudanpioside-h_qt 2 42.36 0.37 DP 28 MOL007384 paeonidanin_qt 65.31 0.35 DP 29 MOL000098 quercetin 46.43 0.28 DP、JYH、HB 30 MOL001454 berberine 36.86 0.78 HB 31 MOL001458 coptisine 30.67 0.86 HB 32 MOL002636 Kihadalactone A 34.21 -

The Role and Robustness of the Gini Coefficient As an Unbiased Tool For
www.nature.com/scientificreports OPEN The role and robustness of the Gini coefcient as an unbiased tool for the selection of Gini genes for normalising expression profling data Marina Wright Muelas 1*, Farah Mughal1, Steve O’Hagan2,3, Philip J. Day3,4* & Douglas B. Kell 1,5* We recently introduced the Gini coefcient (GC) for assessing the expression variation of a particular gene in a dataset, as a means of selecting improved reference genes over the cohort (‘housekeeping genes’) typically used for normalisation in expression profling studies. Those genes (transcripts) that we determined to be useable as reference genes difered greatly from previous suggestions based on hypothesis-driven approaches. A limitation of this initial study is that a single (albeit large) dataset was employed for both tissues and cell lines. We here extend this analysis to encompass seven other large datasets. Although their absolute values difer a little, the Gini values and median expression levels of the various genes are well correlated with each other between the various cell line datasets, implying that our original choice of the more ubiquitously expressed low-Gini-coefcient genes was indeed sound. In tissues, the Gini values and median expression levels of genes showed a greater variation, with the GC of genes changing with the number and types of tissues in the data sets. In all data sets, regardless of whether this was derived from tissues or cell lines, we also show that the GC is a robust measure of gene expression stability. Using the GC as a measure of expression stability we illustrate its utility to fnd tissue- and cell line-optimised housekeeping genes without any prior bias, that again include only a small number of previously reported housekeeping genes. -

Supplementary Figures and Table
SUPPLEMENTARY DATA Supplementary Figure 1. ©2014 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db141 -0066/-/DC1 SUPPLEMENTARY DATA Supplementary Figure 2. ©2014 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db142 -0066/-/DC1 SUPPLEMENTARY DATA -/- Supplementary Table 1. Fold increase of Ser/Thr/Tyr phosphorylation in livers of MKP-3 male mice versus wild type male mice fed on a high fat diet (n=5 for each group). Symbol Name Phosphorylation KO/WT ratio Q Value sites Apoptosis ACIN1 Acin1 protein S64 11.4 0.02 T66 8.3 0.02 API5 Apoptosis inhibitor 5 S461 2.2 0.03 S462 1.8 0.03 AIFM3 Apoptosis-inducing factor 3 S30 7.4 0.03 TP53BP2 Apoptosis-stimulating of p53 protein 2 S479 3.7 0.02 ACIN1 Apoptotic chromatin condensation inducer S64S70 5.7 0.02 1 S208 7.1 0.02 S210 7.0 0.02 S479S482S491 105.7 0.03 S729 2.8 0.02 PEA15 Astrocytic phosphoprotein PEA-15 S116 10.8 0.02 BAG3 BAG family molecular chaperone regulator S179 3.3 0.02 3 S353S357 2.3 0.03 S360 2.3 0.03 S390 8.4 0.02 BNIP2 BCL2/adenovirus E1B 19 kDa-interacting S114 3.9 0.02 protein 2 alpha BNIP3 BCL2/adenovirus E1B 19 kDa protein- S60 19.8 0.03 interacting protein 3 S85T86 14.5 0.02 S88 6.1 0.02 BCL2L13 Bcl-2-like protein 13 S387 4.0 0.02 T389 3.1 0.02 CAAP1 Caspase activity and apoptosis inhibitor S183 2.3 0.03 CARD6 Card6 caspase recruitment domain family, S809 3.6 0.03 member 6 CASP8 Caspase-8 S188 2.2 0.02 DAP Death-associated protein S51 5.4 0.02 DAPK2 Death-associated protein kinase 2 S299 3.8 0.02 S349 3.5 0.02 FAF1 FAS-associated factor 1 S269 17.1 0.04 GAS2 Growth arrest-specific protein 2 T282 5.3 0.02 S283 7.4 0.02 S287 5.3 0.02 S289 7.4 0.02 GCH1 GTP cyclohydrolase 1 S24 3.9 0.02 HTT Huntingtin S398S409S411 9.7 0.02 KRT18 Keratin, type I cytoskeletal 18 T9 2.7 0.02 S31S32S35 2.8 0.02 S43S45 3.1 0.02 PDCD5 MCG128907 S119 10.7 0.02 Y126 4.0 0.02 BNIP3I MCG2480, isoform CRA_b S61S62 12.9 0.03 S63S64 8.1 0.02 ©2014 American Diabetes Association. -

Downloaded from the European Nucleotide Archive (ENA; 126
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 5 September 2018 doi:10.20944/preprints201809.0082.v1 1 Article 2 Transcriptomics as precision medicine to classify in 3 vivo models of dietary-induced atherosclerosis at 4 cellular and molecular levels 5 Alexei Evsikov 1,2, Caralina Marín de Evsikova 1,2* 6 1 Epigenetics & Functional Genomics Laboratory, Department of Molecular Medicine, Morsani College of 7 Medicine, University of South Florida, Tampa, Florida, 33612, USA; 8 2 Department of Research and Development, Bay Pines Veteran Administration Healthcare System, Bay 9 Pines, FL 33744, USA 10 11 * Correspondence: [email protected]; Tel.: +1-813-974-2248 12 13 Abstract: The central promise of personalized medicine is individualized treatments that target 14 molecular mechanisms underlying the physiological changes and symptoms arising from disease. 15 We demonstrate a bioinformatics analysis pipeline as a proof-of-principle to test the feasibility and 16 practicality of comparative transcriptomics to classify two of the most popular in vivo diet-induced 17 models of coronary atherosclerosis, apolipoprotein E null mice and New Zealand White rabbits. 18 Transcriptomics analyses indicate the two models extensively share dysregulated genes albeit with 19 some unique pathways. For instance, while both models have alterations in the mitochondrion, the 20 biochemical pathway analysis revealed, Complex IV in the electron transfer chain is higher in mice, 21 whereas the rest of the electron transfer chain components are higher in the rabbits. Several fatty 22 acids anabolic pathways are expressed higher in mice, whereas fatty acids and lipids degradation 23 pathways are higher in rabbits. -

Elucidating the IGFBP2 Signaling Pathway in Glioma Development and Progression
The Texas Medical Center Library DigitalCommons@TMC The University of Texas MD Anderson Cancer Center UTHealth Graduate School of The University of Texas MD Anderson Cancer Biomedical Sciences Dissertations and Theses Center UTHealth Graduate School of (Open Access) Biomedical Sciences 5-2012 Elucidating the IGFBP2 signaling pathway in glioma development and progression Kristen M. Holmes Follow this and additional works at: https://digitalcommons.library.tmc.edu/utgsbs_dissertations Part of the Cancer Biology Commons, and the Medicine and Health Sciences Commons Recommended Citation Holmes, Kristen M., "Elucidating the IGFBP2 signaling pathway in glioma development and progression" (2012). The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access). 222. https://digitalcommons.library.tmc.edu/utgsbs_dissertations/222 This Dissertation (PhD) is brought to you for free and open access by the The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences at DigitalCommons@TMC. It has been accepted for inclusion in The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences Dissertations and Theses (Open Access) by an authorized administrator of DigitalCommons@TMC. For more information, please contact [email protected]. Elucidating the IGFBP2 signaling pathway in glioma development and progression By Kristen M. Holmes, M.S. APPROVED: ___________________________ Wei Zhang, Ph.D., -

Identification of Human Myometrial Target Genes of the C-Jun NH2
19 Identification of human myometrial target genes of the c-Jun NH2-terminal kinase (JNK) pathway: the role of activating transcription factor 2 (ATF2) and a novel spliced isoform ATF2-small Jarrod Bailey and G Nicholas Europe-Finner School of Surgical and Reproductive Sciences, The Medical School, University of Newcastle upon Tyne, Newcastle upon Tyne NE2 4HH, UK (Requests for offprints should be addressed to J Bailey; Email: [email protected]) Abstract Activating transcription factor 2 (ATF2), a ubiquitously expressed member of the basic region leucine zipper (bZIP) family of transcription factors activated by mitogen activated protein kinase (MAPK) pathways, is important in the mediation of cellular stress responses, development and transformation. We have previously reported the differential expression of active ATF2 in the human myometrium throughout pregnancy and labour, and identified and partially characterized a novel splice variant ATF2-small (ATF2-sm). To further understand the role of these factors in the myometrium, we have used gene microarrays to define the target genes in cultured myometrial cells stably-transfected with ATF2 and ATF2-sm cDNAs. Many of the genes identified appear to have potential roles in regulating myometrial function and include proteins involved in G-protein receptor signalling, cytokine signalling, transcriptional regulation, cell-cycle control, formation of the extracellular matrix and cytoskeletal architecture. ATF2 was found to affect the expression of 204 genes; 113 being up-regulated and 91 down-regulated whereas the novel ATF2-sm factor altered the expression of 55 genes; expression was increased in 29 cases and decreased in 26. A further 25 genes affected by ATF2-sm were identified by suppression subtractive hybridisation (SSH). -

Anti-NACA (C-Terminal) Polyclonal Antibody (DPABH-27484) This Product Is for Research Use Only and Is Not Intended for Diagnostic Use
Anti-NACA (C-terminal) polyclonal antibody (DPABH-27484) This product is for research use only and is not intended for diagnostic use. PRODUCT INFORMATION Antigen Description Prevents inappropriate targeting of non-secretory polypeptides to the endoplasmic reticulum (ER). Binds to nascent polypeptide chains as they emerge from the ribosome and blocks their interaction with the signal recognition particle (SRP), which normally targets nascent secretory peptides to the ER. Also reduces the inherent affinity of ribosomes for protein translocation sites in the ER membrane (M sites) By similarity. Isoform 1 and isoform 2 appear to bind DNA and play roles in transcription. May act to regulate the expression of genes involved in the development of myotubes. Immunogen Synthetic peptide within Mouse Naca-rs (C terminal). The exact sequence is proprietary.Sequence: VMFEERICSESHLGPKAGFRKEKEVNLQELGKVELPPSWFRDLRTKYPVK Isotype IgG Source/Host Rabbit Species Reactivity Mouse Purification Immunogen affinity purified Conjugate Unconjugated Applications WB Format Liquid Size 50 μg Buffer Constituents: 98% PBS, 2% Sucrose Preservative None Storage Shipped at 4°C. Store at 4°C short term (1-2 weeks). Upon delivery aliquot. Store at -20°C long term. Avoid freeze / thaw cycle. GENE INFORMATION 45-1 Ramsey Road, Shirley, NY 11967, USA Email: [email protected] Tel: 1-631-624-4882 Fax: 1-631-938-8221 1 © Creative Diagnostics All Rights Reserved Gene Name NACA nascent polypeptide-associated complex alpha polypeptide [ Mus musculus ] Official Symbol -
Chromosome 12, Frequently Deleted in Human Pancreatic Cancer, May Encode a Tumor-Suppressor Gene That Suppresses Angiogenesis
Laboratory Investigation (2004) 84, 1339–1351 & 2004 USCAP, Inc All rights reserved 0023-6837/04 $30.00 www.laboratoryinvestigation.org Chromosome 12, frequently deleted in human pancreatic cancer, may encode a tumor-suppressor gene that suppresses angiogenesis Sumitaka Yamanaka1,2,*, Makoto Sunamura3,*, Toru Furukawa1, Libo Sun3, Liviu P Lefter1,3, Tadayoshi Abe1,3, Toshimasa Yatsuoka1,3, Hiroko Fujimura3, Emiko Shibuya3, Noriko Kotobuki4, Mitsuo Oshimura4, Akira Sakurada2, Masami Sato2, Takashi Kondo2, Seiki Matsuno3 and Akira Horii1 1Department of Molecular Pathology, Tohoku University School of Medicine, Sendai, Japan; 2Department of Thoracic Surgery, Institute of Development, Aging and Cancer, Tohoku University, Sendai, Japan; 3Department of Gastroenterological Surgery, Tohoku University School of Medicine, Sendai, Japan and 4Department of Cell Technology, Tottori University School of Medicine, Yonago, Japan Several lines of evidence have suggested that the long arm of chromosome 12 may carry a tumor-suppressor gene(s) that plays a role in pancreatic ductal carcinogenesis. We have previously found a significant association between loss of heterozygosity of the 12q arm and a poor prognosis in pancreatic cancer patients. In this study, we introduced a normal copy of chromosome 12 into some pancreatic ductal carcinoma cells. Both anchorage-dependent and -independent proliferations as well as invasiveness were similar throughout the hybrid clones when compared with their corresponding parental cells. In sharp contrast, significant suppression of tumorigenesis was observed after inoculation of the hybrid clones into nude mice. Measurements made up to 1 month later showed that there was a significant delay in the growth of tumors into which the introduced normal copy of chromosome 12 had been restored.