Chaperone Interactions at the Ribosome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Université De La Méditerranée Faculte De Médecine De Marseille École Doctorale Des Sciences De La Vie Et De La Santé Centre De Recherche En Cancérologie De Marseille
UNIVERSITÉ DE LA MÉDITERRANÉE FACULTE DE MÉDECINE DE MARSEILLE ÉCOLE DOCTORALE DES SCIENCES DE LA VIE ET DE LA SANTÉ CENTRE DE RECHERCHE EN CANCÉROLOGIE DE MARSEILLE T H È S E Pour l’obtention du Diplôme de DOCTEUR de L’UNIVERSITÉ de la MÉDITERRANÉE SPÉCIALITÉ : Oncologie : Pharmacologie et Thérapeutique Présentée et soutenue publiquement par Mme Laetitia STUHL-GOURMAND Le 30 Mars 2010 ROLE DE NACA ET DE SES PARTENAIRES MOLÉCULAIRES DANS LA DIFFÉRENCIATION ÉRYTHROÏDE NORMALE ET PATHOLOGIQUE DES SYNDROMES MYÉLODYSPLASIQUES Directeur de Thèse : Dr Sophie GOMEZ Membres du Jury de Thèse : Pr Daniel Olive Président Pr Catherine Lacombe Rapporteur Dr Dominique Duménil Rapporteur Dr Christian Chabannon Examinateur 1 A mes deux hommes, mes deux amours : mon fils Anthony et mon mari Sébastien 2 REMERCIEMENTS Je remercie les membres du jury d’avoir accepté de juger ce travail: les Pr Catherine Lacombe, Dr Christian Chabannon, Dr Dominique Duménil et le Pr Daniel Olive de présider ce jury. Je remercie Françoise Birg de m’avoir accueillie au centre de Recherche en Cancérologie de Marseille UMR891. Je remercie ma directrice de thèse Dr Sophie Gomez a qui je dois beaucoup. Merci de m’avoir toujours soutenue tout au long de cette thèse, et de m’avoir accompagné avec patience dans l’aboutissement de ce travail à mon rythme de jeune maman. Je remercie le Dr Patrice Dubreuil de m’avoir accueilli dans son équipe, d’avoir toujours été à l’écoute, et de m’avoir soutenu pour la finalisation de mon projet. Je remercie Véronique Gelsi-Boyer, Virginie Trouplin, Daniel Birnbaum et Norvert Vey, sans qui le projet MDS n’aurait pas existé. -

Naca Mouse Shrna Plasmid (Locus ID 17938) – TL501429 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TL501429 Naca Mouse shRNA Plasmid (Locus ID 17938) Product data: Product Type: shRNA Plasmids Product Name: Naca Mouse shRNA Plasmid (Locus ID 17938) Locus ID: 17938 Synonyms: AL022831; AL024382; Gm1878; mKIAA0363; skNAC Vector: pGFP-C-shLenti (TR30023) Format: Lentiviral plasmids Components: Naca - Mouse, 4 unique 29mer shRNA constructs in lentiviral GFP vector(Gene ID = 17938). 5µg purified plasmid DNA per construct Non-effective 29-mer scrambled shRNA cassette in pGFP-C-shLenti Vector, TR30021, included for free. RefSeq: BC083340, BC099375, NM_001113199, NM_001282976, NM_013608, BC029830 Summary: Prevents inappropriate targeting of non-secretory polypeptides to the endoplasmic reticulum (ER). Binds to nascent polypeptide chains as they emerge from the ribosome and blocks their interaction with the signal recognition particle (SRP), which normally targets nascent secretory peptides to the ER. Also reduces the inherent affinity of ribosomes for protein translocation sites in the ER membrane (M sites) (By similarity). Isoform 1 and isoform 2 appear to bind DNA and play roles in transcription. Isoform 1 may function as a specific coactivator for JUN, acting to stabilize the interaction of JUN homodimers with promoter elements. [UniProtKB/Swiss-Prot Function] shRNA Design: These shRNA constructs were designed against multiple splice variants at this gene locus. To be certain that your variant of interest is targeted, please contact [email protected]. If you need a special design or shRNA sequence, please utilize our custom shRNA service. -

Post-Translational Modifications and Their Applications in Eye Research (Review)
MOLECULAR MEDICINE REPORTS 15: 3923-3935, 2017 Post-translational modifications and their applications in eye research (Review) BING-JIE CHEN1-3, THOMAS CHUEN LAM3, LONG-QIAN LIU1,2 and CHI-HO TO3 1Department of Optometry and Visual Science, West China Hospital, Sichuan University, Chengdu, Sichuan 610041; 2Institute for Disaster Management and Reconstruction, Sichuan University, Chengdu, Sichuan 610207; 3Laboratory of Experimental Optometry, Centre for Myopia Research, School of Optometry, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong, SAR, P.R. China Received January 9, 2016; Accepted February 22, 2017 DOI: 10.3892/mmr.2017.6529 Abstract. Gene expression is the process by which genetic 3. Introduction of PTMs information is used for the synthesis of a functional gene 4. PTMs in eye research product, and ultimately regulates cell function. The increase 5. Conclusion of biological complexity from genome to proteome is vast, and the post‑translational modification (PTM) of proteins contribute to this complexity. The study of protein expression 1. Introduction and PTMs has attracted attention in the post-genomic era. Due to the limited capability of conventional biochemical Over the last two decades, genomics has been regarded as techniques in the past, large-scale PTM studies were tech- the most popular and productive research field in biological nically challenging. The introduction of effective protein science. However, the intermediate mRNA transcript may separation methods, specific PTM purification strategies rapidly degrade (1) or undergo alternative splicing (2), which and advanced mass spectrometers has enabled the global leads to a number of variable outcomes that renders the study profiling of PTMs and the identification of a targeted PTM of biological systems more challenging. -

Virtual 2-D Map of the Fungal Proteome
www.nature.com/scientificreports OPEN Virtual 2‑D map of the fungal proteome Tapan Kumar Mohanta1,6*, Awdhesh Kumar Mishra2,6, Adil Khan1, Abeer Hashem3,4, Elsayed Fathi Abd‑Allah5 & Ahmed Al‑Harrasi1* The molecular weight and isoelectric point (pI) of the proteins plays important role in the cell. Depending upon the shape, size, and charge, protein provides its functional role in diferent parts of the cell. Therefore, understanding to the knowledge of their molecular weight and charges is (pI) is very important. Therefore, we conducted a proteome‑wide analysis of protein sequences of 689 fungal species (7.15 million protein sequences) and construct a virtual 2‑D map of the fungal proteome. The analysis of the constructed map revealed the presence of a bimodal distribution of fungal proteomes. The molecular mass of individual fungal proteins ranged from 0.202 to 2546.166 kDa and the predicted isoelectric point (pI) ranged from 1.85 to 13.759 while average molecular weight of fungal proteome was 50.98 kDa. A non‑ribosomal peptide synthase (RFU80400.1) found in Trichoderma arundinaceum was identifed as the largest protein in the fungal kingdom. The collective fungal proteome is dominated by the presence of acidic rather than basic pI proteins and Leu is the most abundant amino acid while Cys is the least abundant amino acid. Aspergillus ustus encodes the highest percentage (76.62%) of acidic pI proteins while Nosema ceranae was found to encode the highest percentage (66.15%) of basic pI proteins. Selenocysteine and pyrrolysine amino acids were not found in any of the analysed fungal proteomes. -

Role of CCT Chaperonin in the Disassembly of Mitotic Checkpoint Complexes
Role of CCT chaperonin in the disassembly of mitotic checkpoint complexes Sharon Kaisaria, Danielle Sitry-Shevaha, Shirly Miniowitz-Shemtova, Adar Teichnera, and Avram Hershkoa,1 aUnit of Biochemistry, Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa 31096, Israel Contributed by Avram Hershko, December 14, 2016 (sent for review November 23, 2016; reviewed by Michele Pagano and Alexander Varshavsky) The mitotic checkpoint system prevents premature separation of thebindingoftheATPasetoMCC(13,14).TheenergyofATP sister chromatids in mitosis and thus ensures the fidelity of hydrolysis is apparently used in this process to promote a confor- chromosome segregation. When this checkpoint is active, a mitotic mational change of Mad2 back from C-Mad2 to O-Mad2, leading checkpoint complex (MCC), composed of the checkpoint proteins to loss of its affinity for Cdc20 in MCC and thus to Mad2 release. Mad2, BubR1, Bub3, and Cdc20, is assembled. MCC inhibits the By the action of TRIP13 and p31comet, MCC is converted to a ubiquitin ligase anaphase promoting complex/cyclosome (APC/C), remnant, called BC-1, in which Cdc20 is still associated with whose action is necessary for anaphase initiation. When the check- BubR1 (15). Cdc20 is released from BubR1 in MCC by a dif- point signal is turned off, MCC is disassembled, a process required ferent process involving the phosphorylation of Cdc20 by Cdk1 for exit from checkpoint-arrested state. Different moieties of MCC (16). We noticed, however, that a considerable part of the release are disassembled by different ATP-requiring processes. Previous of Cdc20 from MCC was due to a phosphorylation-independent, work showed that Mad2 is released from MCC by the joint action comet but ATP-dependent process (16). -

Crystal Structures of NAC Domains of Human Nascent Polypeptide-Associated Complex (NAC) and Its Αnac Subunit
Protein Cell 2010, 1(4): 406–416 Protein & Cell DOI 10.1007/s13238-010-0049-3 RESEARCH ARTICLE Crystal structures of NAC domains of human nascent polypeptide-associated complex (NAC) and its αNAC subunit ✉ Lanfeng Wang1, Wenchi Zhang1, Lu Wang1, Xuejun C.Zhang1, Xuemei Li1, Zihe Rao1,2,3 1 National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, 15 Datun Road, Beijing 100101, China 2 Structure Biology Laboratory, Tsinghua University, Beijing 100084, China 3 Tianjin Key Laboratory of Protein Science, College of Life Sciences, Nankai University, Tianjin 300071, China ✉ Correspondence: [email protected] Received March 28, 2010 Accepted April 12, 2010 ABSTRACT binding with other cytosolic factors (Wiedmann et al., 1994). This complex is widely conserved from archaea to human. Nascent polypeptide associated complex (NAC) and its Some NAC mutants induce early embryonically lethal two isolated subunits, αNAC and βNAC, play important phenotypes in mice, fruit fly, and Caenorhabditis elegans roles in nascent peptide targeting. We determined a 1.9 Å (Deng and Behringer, 1995; Markesich et al., 2000). Experi- resolution crystal structure of the interaction core of NAC ments to determine NAC intracellular localization and heterodimer and a 2.4 Å resolution crystal structure of distribution show that the vast majority of NAC is in the αNAC NAC domain homodimer. These structures provide cytoplasm as a stable heterodimer, and no single subunit and detailed information of NAC heterodimerization and homo-oligomer has been observed under physiologic condi- αNAC homodimerization. We found that the NAC tions (Beatrix et al., 2000). However, drastic variations of domains of αNAC and βNAC share very similar folding relative concentration between the two NAC subunits are despite of their relative low identity of amino acid found in patients of Alzheimer’s disease, Down syndrome, sequences. -

An Ensemble of Cryo-EM Structures of Tric Reveal Its Conformational Landscape and Subunit Specificity
An ensemble of cryo-EM structures of TRiC reveal its conformational landscape and subunit specificity Mingliang Jina, Wenyu Hana, Caixuan Liua, Yunxiang Zanga, Jiawei Lia, Fangfang Wanga,b, Yanxing Wanga,b, and Yao Conga,b,1 aNational Center for Protein Science Shanghai, State Key Laboratory of Molecular Biology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China; and bShanghai Science Research Center, Chinese Academy of Sciences, Shanghai 201210, China Edited by Wolfgang Baumeister, Max Planck Institute of Biochemistry, Martinsried, Germany, and approved August 14, 2019 (received for review March 9, 2019) TRiC/CCT assists the folding of ∼10% of cytosolic proteins through neighboring subunit, forming a “hand-in-hand” intra-ring interac- an ATP-driven conformational cycle and is essential in maintaining tion structural signature, which plays an essential role in intra-ring protein homeostasis. Here, we determined an ensemble of cryo- allosteric network (20). electron microscopy (cryo-EM) structures of yeast TRiC at various Extensive efforts have been made to study the structures of nucleotide concentrations, with 4 open-state maps resolved at archaeal group II chaperonins (21–23), as well as the eukaryotic near-atomic resolutions, and a closed-state map at atomic resolu- group II chaperonin TRiC, by crystallography (24, 25) and cryo- tion, revealing an extra layer of an unforeseen N-terminal alloste- electron microscopy (cryo-EM) (4, 5, 19, 26–28). Recently, we ric network. We found that, during TRiC ring closure, the CCT7 resolved 2 cryo-EM structures of Saccharomyces cerevisiae TRiC subunit moves first, responding to nucleotide binding; CCT4 is in a newly identified nucleotide partially preloaded state (NPP- the last to bind ATP, serving as an ATP sensor; and CCT8 remains ADP-bound and is hardly involved in the ATPase-cycle in our ex- TRiC) and in the ATP-bound state (TRiC-AMP-PNP) (29). -

Efficiency of Cleavage at the Polyadenylation Site MOSHE SADOFSKY and JAMES C
MOLECULAR AND CELLULAR BIOLOGY, Aug. 1984, p. 1460-1468 Vol. 4. No. 8 0270-7306/84/081460-09$02.00/0 Copyright ©D 1984, American Society for Microbiology Sequences on the 3' Side of Hexanucleotide AAUAAA Affect Efficiency of Cleavage at the Polyadenylation Site MOSHE SADOFSKY AND JAMES C. ALWINE* Department of Microbiology, School of Medicine/G2, UnivSersity of Pennsylvania, Phtiladelphia, Pennsylvania /9104 Received 3 April 1984/Accepted 8 May 1984 The hexanucleotide AAUAAA has been demonstrated to be part of the signal for cleavage and polyadenyla- tion at appropriate sites on eucaryotic mRNA precursors. Since this sequence is not unique to polyadenylation sites, it cannot be the entire signal for the cleavage event. We have extended the definition of the polyadenylation cleavage signal by examining the cleavage event at the site of polyadenylation for the simian virus 40 late mRNAs. Using viable mutants, we have determined that deletion of sequences between 3 and 60 nucleotides on the 3' side of the AAUAAA decreases the efficiency of utilization of the normal polyadenylation site. These data strongly indicate a second major element of the polyadenylation signal. The phenotype of these deletion mutants is an enrichment of viral late transcripts longer than the normally polyadenylated RNA in infected cells. These extended transcripts appear to have an increased half-life due to the less efficient cleavage at the normal polyadenylation site. The enriched levels of extended transcripts in cells infected with the deletion mutants allowed us to examine regions of the late transcript which normally are difficult to study. The extended transcripts have several discrete 3' ends which we have analyzed in relation to polyadenylation and other RNA processing events. -

Discovery of RSV-Induced BRD4 Protein Interactions Using Native Immunoprecipitation and Parallel Accumulation – Serial Fragmentation (PASEF) Mass Spectrometry
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 26 February 2021 Article Discovery of RSV-induced BRD4 protein interactions using native immunoprecipitation and parallel accumulation – serial fragmentation (PASEF) mass spectrometry Morgan Mann 1 , David Roberts 2 , Yanlong Zhu 3,4 , Yi Li 5, Jia Zhou 5 , Ying Ge 2,3,4 and Allan R. Brasier 6* 1 Department of Medicine, Division of Endocrinology, Diabetes, and Metabolism, University of Wisconsin-Madison, Madison, WI, 53705, USA 2 Department of Chemistry, University of Wisconsin-Madison, Madison, WI, 53706, USA 3 Human Proteomics Program, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, 53705, USA 4 Department of Cell and Regenerative Biology, University of Wisconsin-Madison, Madison, WI, 53705, USA 5 Department of Pharmacology and Toxicology, University of Texas Medical Branch, Galveston, TX 77550 6 Institute for Clinical and Translational Research (ICTR), University of Wisconsin-Madison, Madison, WI, 53705, USA * Correspondence: [email protected]; Tel.: 1-608-263-7371 1 Abstract: Respiratory Syncytial Virus (RSV) causes severe inflammation and airway pathology 2 in children and the elderly by infecting the epithelial cells of the upper and lower respiratory 3 tract. RSV replication is sensed by intracellular pattern recognition receptors upstream of the IRF 4 and NF-κB transcription factors. These proteins coordinate an innate inflammatory response via 5 Bromodomain containing protein 4 (BRD4), a protein that functions as a scaffold for unknown 6 transcriptional regulators. To better understand the pleiotropic regulatory function of BRD4, 7 we examine the BRD4 interactome and identify how RSV infection dynamically alters it. To 8 accomplish these goals, we leverage native immunoprecipitation and Parallel Accumulation – 9 Serial Fragmentation (PASEF) mass spectrometry to examine BRD4 complexes isolated from 10 human alveolar epithelial cells in the absence or presence of RSV infection. -
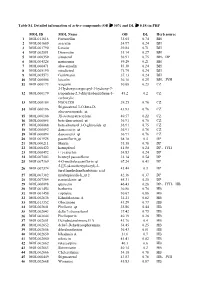
MOL ID MOL Name OB DL Herb Source 1 MOL011616 Fortunellin
Table S1. Detailed information of active compounds (OB ≥ 30% and DL ≥ 0.18) in PHF MOL ID MOL Name OB DL Herb source 1 MOL011616 Fortunellin 35.65 0.74 BH 2 MOL001689 acacetin 34.97 0.24 BH 3 MOL001790 Linarin 39.84 0.71 BH 4 MOL002881 Diosmetin 31.14 0.27 BH 5 MOL000359 sitosterol 36.91 0.75 BH、DP 6 MOL004328 naringenin 59.29 0.21 BH 7 MOL000471 aloe-emodin 83.38 0.24 BH 8 MOL005190 eriodictyol 71.79 0.24 BH 9 MOL005573 Genkwanin 37.13 0.24 BH 10 MOL000006 luteolin 36.16 0.25 BH、JYH 11 MOL000173 wogonin 30.68 0.23 CZ 2-Hydroxyisoxypropyl-3-hydroxy-7- 12 MOL000179 isopentene-2,3-dihydrobenzofuran-5- 45.2 0.2 CZ carboxylic 13 MOL000184 NSC63551 39.25 0.76 CZ Stigmasterol 3-O-beta-D- 14 MOL000186 43.83 0.76 CZ glucopyranoside_qt 15 MOL000188 3β-acetoxyatractylone 40.57 0.22 CZ 16 MOL000085 beta-daucosterol_qt 36.91 0.75 CZ 17 MOL000088 beta-sitosterol 3-O-glucoside_qt 36.91 0.75 CZ 18 MOL000092 daucosterin_qt 36.91 0.76 CZ 19 MOL000094 daucosterol_qt 36.91 0.76 CZ 20 MOL001925 paeoniflorin_qt 68.18 0.4 DP 21 MOL000211 Mairin 55.38 0.78 DP 22 MOL000422 kaempferol 41.88 0.24 DP、JYH 23 MOL000492 (+)-catechin 54.83 0.24 DP 24 MOL007003 benzoyl paeoniflorin 31.14 0.54 DP 25 MOL007369 4-O-methylpaeoniflorin_qt 67.24 0.43 DP 5-[[5-(4-methoxyphenyl)-2- 26 MOL007374 43.44 0.3 DP furyl]methylene]barbituric acid 27 MOL007382 mudanpioside-h_qt 2 42.36 0.37 DP 28 MOL007384 paeonidanin_qt 65.31 0.35 DP 29 MOL000098 quercetin 46.43 0.28 DP、JYH、HB 30 MOL001454 berberine 36.86 0.78 HB 31 MOL001458 coptisine 30.67 0.86 HB 32 MOL002636 Kihadalactone A 34.21 -

Cellular Proteomes Drive Tissue-Specific Regulation of The
INVESTIGATION Cellular Proteomes Drive Tissue-Specific Regulation of the Heat Shock Response Jian Ma,1 Christopher E. Grant,1 Rosemary N. Plagens, Lindsey N. Barrett, Karen S. Kim Guisbert, and Eric Guisbert2 Department of Biological Sciences, Florida Institute of Technology, Melbourne, Florida 32901 ORCID ID: 0000-0003-1901-5598 (E.G.) ABSTRACT The heat shock response (HSR) is a cellular stress response that senses protein misfolding and KEYWORDS restores protein folding homeostasis, or proteostasis. We previously identified an HSR regulatory network in stress response Caenorhabditis elegans consisting of highly conserved genes that have important cellular roles in main- protein folding taining proteostasis. Unexpectedly, the effects of these genes on the HSR are distinctly tissue-specific. Here, proteostasis we explore this apparent discrepancy and find that muscle-specific regulation of the HSR by the TRiC/CCT HSF1 chaperonin is not driven by an enrichment of TRiC/CCT in muscle, but rather by the levels of one of its most heat shock abundant substrates, actin. Knockdown of actin subunits reduces induction of the HSR in muscle upon TRiC/ response CCT knockdown; conversely, overexpression of an actin subunit sensitizes the intestine so that it induces the HSR upon TRiC/CCT knockdown. Similarly, intestine-specific HSR regulation by the signal recognition particle (SRP), a component of the secretory pathway, is driven by the vitellogenins, some of the most abundant secretory proteins. Together, these data indicate that the specific protein folding requirements from the unique cellular proteomes sensitizes each tissue to disruption of distinct subsets of the proteostasis network. These findings are relevant for tissue-specific, HSR-associated human diseases such as cancer and neurodegenerative diseases. -

The Chaperonin Tric/CCT Is Essential for the Action of Bacterial Glycosylating Protein Toxins Like Clostridium Difficile Toxins a and B
The chaperonin TRiC/CCT is essential for the action of bacterial glycosylating protein toxins like Clostridium difficile toxins A and B Marcus Steinemanna,b, Andreas Schlosserc, Thomas Janka,1, and Klaus Aktoriesa,d,1 aInstitute for Experimental and Clinical Pharmacology and Toxicology, Faculty of Medicine, University of Freiburg, 79104 Freiburg, Germany; bFaculty of Biology, University of Freiburg, 79104 Freiburg, Germany; cRudolf Virchow Center for Experimental Biomedicine, University of Würzburg, 97080 Würzburg, Germany; and dCenter for Biological Signaling Studies, University of Freiburg, 79104 Freiburg, Germany Edited by F. Ulrich Hartl, Max Planck Institute of Biochemistry, Martinsried, Germany, and approved August 8, 2018 (received for review May 3, 2018) Various bacterial protein toxins, including Clostridium difficile tox- however, how the large toxins cross the endosomal membrane and ins A (TcdA) and B (TcdB), attack intracellular target proteins of host how the glucosyltransferase is reactivated in the cytosol remain cells by glucosylation. After receptor binding and endocytosis, the unknown. Moreover, the question of reactivation and refolding of toxins are translocated into the cytosol, where they modify target bacterial glycosyltransferases during and after translocation is proteins (e.g., Rho proteins). Here we report that the activity of enigmatic for many toxins/effectors known. In this study, we found translocated glucosylating toxins depends on the chaperonin that chaperonin TCP-1 ring complex (TRiC)/ chaperonin con- TRiC/CCT. The chaperonin subunits CCT4/5 directly interact with taining TCP-1 (CCT) plays a pivotal role in toxin translocation the toxins and enhance the refolding and restoration of the gluco- and/or refolding. TRiC/CCT is a molecular machine involved in syltransferase activities of toxins after heat treatment.