The Role of Vision in Sexual Signaling in the Blue Crab by Jamie Baldwin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sydney Harbour: What We Do and Do Not Know About a Highly Diverse Estuary
Marine and Freshwater Research 2015, 66, 1073-1087 © CSIRO 2015 http://dx.doi.org/10.1071/MF15159_AC Supplementary material Sydney Harbour: what we do and do not know about a highly diverse estuary E. L. JohnstonA,B, M. Mayer-PintoA,B, P. A. HutchingsC, E. M. MarzinelliA,B,D, S. T. AhyongC, G. BirchE, D. J. BoothF, R. G. CreeseG, M. A. DoblinH, W. FigueiraI, P. E. GribbenB,D, T. PritchardJ, M. RoughanK, P. D. SteinbergB,D and L. H. HedgeA,B AEvolution and Ecology Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. BSydney Institute of Marine Science, 19 Chowder Bay Road, Mosman, NSW 2088, Australia. CAustralian Museum Research Institute, Australian Museum, 6 College Street, Sydney, NSW 2010, Australia. DCentre for Marine Bio-Innovation, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. ESchool of GeoSciences, The University of Sydney, Sydney, NSW 2006, Australia. FCentre for Environmental Sustainability, School of the Environment, University of Technology, Sydney, NSW 2007, Australia. GNew South Wales Department of Primary Industries, Port Stephens Fisheries Institute, Nelson Bay, NSW 2315, Australia. HPlant Functional Biology and Climate Change Cluster, University of Technology, Sydney, NSW 2007, Australia. ICentre for Research on Ecological Impacts of Coastal Cities, School of Biological Sciences, University of Sydney, NSW 2006, Australia. JWater and Coastal Science Section, New South Wales Office of Environment and Heritage, PO Box A290, Sydney, NSW 1232, Australia. KCoastal and Regional Oceanography Lab, School of Mathematics and Statistics, University of New South Wales, NSW 2052, Australia. -

Archiv Für Naturgeschichte
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Bericht über die Leistuugen in der Carcinologie wälirend des Jahres 1894. Von Dr. F. Hilgendorf und Dr. J. Vosseier*). Verzeichniss der Publicationen. Albert I,, Prince de Monaco: Sur les premieres campagnes de la princesse Alice. Compt. rend. Ac. Sc. Paris T. CXX. — Eine 2 m tief ins Meer eingesenkte Lampe lockt in kurzer Zeit ganze Wolken kleiner Kruster herbei. F. Albrecht, L. K., Ziornow u. a. Primitiae faunae Mosquensis. Congres intern, d'anthrop., arch. et zool. 1892 (Moscou), Materiaux i-eunis etc. 1. partie, Suppl. Nr. 16, 137 S.; Crust. p. 121 — 5. Mos- cou 1893. — 122 Entom. u. 14 Malacostraca, Alcock, A. Natural bist, notes from „Investigator" (Ser. 2) Nr. 1. (continued). (Vergl. Ber. 91, 92, 93 unter Wood-Mason, W.- M. u. Alcock, Alcock). Ann. Mag. (6) XIII p. 225-45, 321—34, 400—411. — Behandelt Deep-sea dredging 1890/91. Spec. Nr. 58 bis 99. Farn. Nematocarcinidae, Honiar., Eryont. (IXyl.), Parapagur., Galath., Inachidae, Cancridae (Platypilumyins)^ Ocypod. (Psopheticus), Leucos. {Ci/monomops), Homolidae. Stomatopoda (2 Sp.), Amphi- poda (l Sp., Farn. Stegoceph., Xyl). 28 neue Sp. od. Variet. Sperma- tozoen V. Munida besclir. p. 324. Stridulationsapp. bei Psophet. Rudim. Augen bei Cymon. u. Andania. Alcock, A. and A. R. Audeison (1). Nat. bist, notes from „Investigator" (2. Ser.) No. 14: An account of a recent coli, of deep sea Crustacea from the Bay of Bengal and Laccadive Sea. Journ. Asiat, soc. of Bengal, Vol. 63 part. IL No. 3. p. 141—185. Tfl. IX. *) Im Allgemeinen sind die Arbeiten über höhere Krebse von Hilgendorf, die über niedere von Vosseier besprochen worden, lieber etwaige Ausnahmen giebt die Unterzeichnung der betreif. -

Download Full Article 1.3MB .Pdf File
Memoirs of the National Museum of Victoria 12 April 1971 Port Phillip Bay Survey 2 https://doi.org/10.24199/j.mmv.1971.32.05 BRACHYURA (CRUSTACEA, DECAPODA) By D. J. G. Griffin and J. C. Yaldwyn* Australian Museum, Sydney Abstract The SurVey C0 Iected 102 specimens of Brachyura *a -| c ! ? belonging to 29 Species and 10 families.m Seven species were taken by the Portland Pier Survey in 1963 five of which are also represented in the Port Phillip Survey collection. Only four of the 38 species known m 3re re resent d the collection. P ? '? The majid Paratymolus talipes and the xanthidTamh-YPilumnuspf acer are recorded from Victoria for the first time; previous records of the graspid\Cyclograpsus audouinii from Victoria are doubtful. Seventeen species known from Port Phillip are not represented in the collection. All are typically cool temperate species well known from SE. Australia. Four species of Pilumnus were represented in the collections and these are compared in detail with other SE. Australian Pilumnus species. Most abundant in Port Phillip are Hahcaranus ovatus and H. rostratus (Hymenosomatidae) Notomithrax minor (Majidae), Ebalia (Phylyxia) intermedia (Leucosiidae), Lilocheira bispinosa (Gone- placidae), Pilumnus tomentosus and P. monilifer (Xanthidae), Nectocardnus integrifrons and Carcinus maenas (Portunidae) and Pinnotheres pisum (Pinnotheridae). The majority of the species are found on the sandy areas around the edge of the Bay, particularly in the W areas; no species was taken in the central deeper parts of the Bay. Ovigerous females of most species were collected in late summer. Parasitism by sacculinas was small and confined to two species of Pilumnus. -

Adaptations by the Locomotor Systems of Terrestrial and Amphibious Crabs Walking Freely on Land and Underwater
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2004 Adaptations by the locomotor systems of terrestrial and amphibious crabs walking freely on land and underwater Jennifer Nuss Schreiner Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Recommended Citation Schreiner, Jennifer Nuss, "Adaptations by the locomotor systems of terrestrial and amphibious crabs walking freely on land and underwater" (2004). LSU Master's Theses. 1349. https://digitalcommons.lsu.edu/gradschool_theses/1349 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. ADAPTATIONS BY THE LOCOMOTOR SYSTEMS OF TERRESTRIAL AND AMPHIBIOUS CRABS WALKING FREELY ON LAND AND UNDERWATER A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in The Department of Biological Sciences by Jennifer Nuss Schreiner B.S., Louisiana State University, 2001 August 2004 ACKNOWLEDGEMENTS I would like to begin by expressing my most heartfelt appreciation to Dr. Jim Belanger. Thank you for giving me the opportunity to be part of your laboratory family, for your everlasting faith in me, and for the endless hours of reassurance when it seemed nothing would ever go as planned. Your patience and generosity mean a great deal to me. -
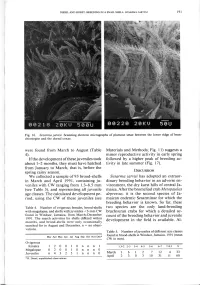
(Table 4). If the Development of These Juveniles Took About 1-2 Months
DIESEL AND HORST: BREEDING IN A SNAIL SHELL: SESARMA JARVISI 191 Fig. 16. Sesarma jarvisi. Scanning electron micrographs of plumose setae between the lower ridge of bran- chiostegite and the dorsal coxae. were found from March to August (Table Materials and Methods; Fig. 11) suggests a 4). minor reproductive activity in early spring If the development of these juveniles took followed by a higher peak of breeding ac- about 1-2 months, they must have hatched tivity in late summer (Fig. 17). from January to March, that is, before the spring rainy season. DISCUSSION We collected a sample of 93 brood-shells Sesarma jarvisi has adopted an extraor- in March and April 1991, containing ju- dinary breeding behavior in an adverse en- veniles with CW ranging from 1.3-8.5 mm vironment, the dry karst hills of central Ja- (see Table 5), and representing all juvenile maica. After the bromeliad crab Metopaulias age classes. The calculated development pe- depressus, it is the second species of Ja- riod, using the CW of these juveniles (see maican endemic Sesarminae for which the breeding behavior is known. So far, these Table 4. Number of ovigerous females, brood-shells two species are the only land-breeding with megalopae, and shells with juveniles <3-mm CW brachyuran crabs for which a detailed ac- found in Windsor, Jamaica, from March-December count of the breeding behavior and juvenile 1991. The search activities for shells differed within months, and brood-shells were only occasionally development in the field is available. Al- searched for in August and December, n = no obser- vations. -

Behavioural and Molecular Evidence for the Systematic Position of Macrophthalmus (Hemiplax) Hirtipes Hombron & Jacquinot, 18
BEHAVIOURAL AND MOLECULAR EVIDENCE FOR THE SYSTEMATIC POSITION OF MACROPHTHALMUS (HEMIPLAX) HIRTIPES HOMBRON & JACQUINOT, 1846, WITH COMMENTS ON MACROPHTHALMINE SUBGENERA (DECAPODA, BRACHYURA, MACROPHTHALMIDAE) BY COLIN L. MCLAY1,3), JUN KITAURA2) and KEIJI WADA2) 1) School of Biological Sciences, Canterbury University, Christchurch, 8004, New Zealand 2) Department of Biological Science, Faculty of Science, Nara Women’s University, Kitauoya-nishimachi, Nara 630-8506, Japan ABSTRACT The analysis of 16s rRNA data from the New Zealand sentinel crab, Macrophthalmus (Hemiplax) hirtipes (Jacquinot & Hombron, 1846) (Macrophthalminae: Macrophthalmidae) shows that it is the most basal species in the genus Macrophthalmus, and behavioural trait mapping suggests that ancestral behaviours included extended cheliped fighting. Other behaviour found in some sentinel crabs, such as allocleaning and waving displays, were absent in the ancestor and are regarded here as derived traits. It is proposed that the two subgenera Hemiplax and Tasmanoplax be elevated to full generic status. Three subgenera appear to be polyphyletic: Macrophthalmus (26 species), Mareotis (14 species) and Paramareotis (4 species), and to resolve this problem molecular data from the remaining species will need to be added into the analysis. The Australasian fauna contains the four most basal genera of the Macrophthalminae: Tasmanoplax, Hemiplax, Australoplax,andEnigmaplax. Our hypothesis is that these were early off-shoots from Tethyan ancestor(s) that colonized Australasia. RÉSUMÉ L’analyse des données d’ARNr 16S du crabe sentinelle venant de la néo-zélandais, Macroph- thalmus (Hemiplax) hirtipes (Jacquinot et Hombron, 1846) (Macrophthalminae: Macrophthal- midae) montre que cette espèce est la plus basale du genre Macrophthalmus, et l’analyse des traits comportementaux suggère que le comportements de ses les ancêtres incluaient des com- bats avec les chélipédes déployés. -

Number of Ghost Crab Burrows Does Not Correspond to Population Size
Cent. Eur. J. Biol. • 8(9) • 2013 • 843-847 DOI: 10.2478/s11535-013-0208-7 Central European Journal of Biology Number of ghost crab burrows does not correspond to population size Communication Willian T.A.F. Silva1,2,*, Tereza C.S. Calado1 1Integrated Laboratories of Marine and Natural Sciences, Federal University of Alagoas, 57051-090 Maceió-AL, Brazil 2Institute of Evolutionary Sciences of Montpellier, Group of Developmental and Evolutionary Biology, University of Montpellier 2, 34095 Montpellier, France Received 12 December 2012; Accepted 08 May 2013 Abstract: Ghost crabs are distributed worldwide on sandy beaches, and several studies have associated the number of ghost crab burrows with the levels of anthropogenic impacts on the beaches under study. However, our results show that the use of ghost crab Ocypode quadrata burrows to assess levels of anthropogenic impacts on sand beaches may not be accurate, as previously thought, because the number of burrows does not represent an estimate of the population size. In addition, we propose three hypotheses to explain the extremely low number of individuals/number of burrows ratio: the “secret chamber”, the “multiple openings”, and the “one crab, several burrows” hypotheses. We also observed an unusual sex ratio. Keywords: Ocypode quadrata • Ocypodidae • Sandy beaches • Anthropogenic impacts © Versita Sp. z o.o. 1. Introduction [12-15]. In a field survey, we tested the hypothesis that the number of ghost crab O. quadrata burrows on sandy The ghost crab Ocypode quadrata (Fabricius, 1787), beaches corresponds to the estimated number of crabs also known as maria-farinha in Brazil, is the only in the population. -

Spatial Distribution and Substratum
SPATIAL DISTRIBUTION AND SUBSTRATUM PREFERENCE OF THE BRACHYURAN CRAB, MACROPHTHALMUS DEPRESSUS (DECAPODA, OCYPODIDAE) ALONG THE LOWER ESTUARINE MUDFLAT OF MAHI RIVER, GUJARAT, INDIA BY PRANAV J. PANDYA and KAURESH D. VACHHRAJANI1) Division of Environment and Toxicology, Department of Zoology, Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara 390002, Gujarat, India ABSTRACT The distribution of the ocypodid crab, Macrophthalmus depressus Rüppell, 1830, was studied on the lower intertidal mudflats of the Mahi River estuary of the Gulf of Khambhat, Gujarat, India. Zoning of the study area was done based on coastal morphology and sediment composition. Distribution pattern and density were studied using transect and quadrate sampling methods. Five zones with variable sand, silt, and clay composition could be distinguished perpendicular to the shore line. Zones 1 and 2 represented the upper inter tidal area (60% sand), Zones 3 and 4 the mid-inter tidal area (70-80% silt + clay) and Zone 5 the lower inter tidal area (50% sand). Quadrant analysis showed the density of M. depressus burrows as follows: Zone 1, no animals present (hence not further included in the study); Zone 2, 28.66 ± 9.86; Zone 3, 83.20 ± 33.87; Zone 4, 62.00 ± 9.16; and Zone 5, 52.50±24.74 individuals per m2. The mean density of the crab varied from 0 to 83 individuals per linear metre along the transect all over the above mentioned zones. The densier distribution in, and thus presumed preference for, Zones 3 and 4 can be attributed to the sediment composition as well as to the slope, which together make the substrate firm and suitable for easy burrow construction. -

Visual Ecology of the Fiddler Crab, Uca Tangeri
ANIMAL BEHAVIOUR, 2008, 75, 175e188 doi:10.1016/j.anbehav.2007.04.016 Available online at www.sciencedirect.com Visual ecology of the fiddler crab, Uca tangeri: effects of sex, viewer and background on conspicuousness MOLLY E. CUMMINGS*,JOANAM.JORDAO~ †,THOMASW.CRONIN‡ &RUIF.OLIVEIRA† *Section of Integrative Biology, University of Texas at Austin yUnidade de Investigacao em Eco-Etologia, Instituto Superior de Psicologia Aplicada, Lisboa, Portugal zDepartment of Biology, University of Maryland at Baltimore County, U.S.A. (Received 19 June 2006; initial acceptance 24 September 2006; final acceptance 9 April 2007; MS. number: A10479R) We investigated the visual ecology of the coloration of the eastern Atlantic fiddler crab, Uca tangeri, with particular attention to predator (e.g. avian) and conspecific vision. Spectral reflectance measurements were made on different body parts used in possible intraspecific communication as well as background habitats including crab-made materials (e.g. mudballs). Avian-based and crab-based visual models were used to ob- tain different estimates of crab conspicuousness to potential predators and conspecifics. We found that male body parts (except for dorsal carapace) were significantly more conspicuous to conspecific viewers than female equivalent body parts, and showed greater within-body contrast estimates. Moreover, male major claw areas differed in reflectance properties, producing variation in conspicuousness that fit signal- ling predictions: areas visible during claw-waving events were most conspicuous against the background sky, whereas areas visible in nonwaving positions were more conspicuous against substrate backgrounds. For avian vision, sexually dimorphic coloration results in males being generally more conspicuous than females (in terms of brightness contrast) against all backgrounds, however, there was no sexual dimorphic conspicuousness of carapace coloration. -

Accumulation of Metals by Toadfish from Sediment and Infauna: Are Fish What They Eat?
AUSTRALASIAN JOURNAL OF ECOTOXICOLOGY Vol. 12, pp. 95-106, 2006 Metal accumulation by toadfish and prey Alquezar and Markich PAPERS ACCUMULATION OF METALS BY TOADFISH FROM SEDIMENT AND INFAUNA: ARE FISH WHAT THEY EAT? Ralph Alquezar1, 2, 3* and Scott J Markich4 1 Department of Environmental Sciences, University of Technology Sydney, PO Box 123, Broadway 2007, NSW, Australia. 2 Institute for Environmental Research, Australian Nuclear Science and Technology Organisation, PMB 1, Menai 2234, NSW, Australia. 3 Current address: Centre for Environmental Management, Central Queensland University, PO Box 1319, Gladstone 4680, Queensland, Australia. 4 Aquatic Solutions International, Level 1, 467 Miller St, Cammeray 2062, NSW, Australia Manuscript received, 16/9/2006; accepted, 2/11/2006. ABSTRACT Metals may have direct and indirect effects on aquatic biota at different trophic levels. This study examined the metal concentrations and nutritional value (protein and lipid content) of sediment infauna consumed by the estuarine smooth toadfish (Tetractenos glaber) at sites with varying metal contamination in the Parramatta River (Sydney Harbour), south- eastern Australia, and the resulting influence on toadfish size and their tissue metal concentrations. Metal concentrations in sediments showed positive linear relationships (r2 = 0.29–0.87; P < 0.001, n = 12) with metal concentrations in sediment infauna. Metal concentrations in toadfish tissues were also linearly and positively related to metal concentrations in both sediments (r2 = 0.32–0.73; P < 0.001, n = 55) and infauna (r2 = 0.27–0.72; P < 0.001, n = 55), indicating that sediment and infauna are an important metal exposure pathway for toadfish. Of the key toadfish prey items (sediment infauna), polychaetes (Marphysa sanguinea) generally had the highest metal concentrations and nutritional value followed by semaphore crabs (Heloecius cordiformis) and black mussels (Xenostrobus securis). -

Morphological Diversity of Setae and Chela in Accordance to Habitat Within Crabs of Genus Macrophthalmus
Morphological diversity of setae and chela in accordance to habitat within crabs of genus Macrophthalmus (Decapoda, Brachyura, Macrophthalmidae) from intertidal flats of Pakistan Item Type article Authors Aziz, Uroj; Saher, Noor Us Download date 29/09/2021 07:03:55 Link to Item http://hdl.handle.net/1834/40805 Pakistan Journal of Marine Sciences, Vol. 25(1&2), 83-91, 2016. MORPHOLOGICAL DIVERSITY OF SETAE AND CHELA IN ACCORDANCE TO HABITAT WITHIN CRABS OF GENUS MACROPHTHALMUS (DECAPODA, BRACHYURA, MACROPHTHALMIDAE) FROM INTERTIDAL FLATS OF PAKISTAN Uroj Aziz and Noor Us Saher APWA Govt. College for Women Karimabad, Karachi (UA); Centre of Excellence in Marine Biology, University of Karachi (NUS). email: [email protected] ABSTRACT: Sentinel crabs of genus Macrophthalmus are deposit feeders inhabiting sandy muddy substrate. The diversity and distribution of these crabs depend upon physical, biological and environmental factors including tidal exposure, larval recruitment, salinity, pH, organic matters, air and water temperature, nutrient availability and sediment structure in which they survive. These species showed various morphological adaptations to cope with their environment and to extract food from their surroundings. Current study aimed to study structural variation in 2nd maxilliped and chela and correlate it with their habitat. The substrate structure appears as one of the most important controlling factor in the distribution of sentinel crabs. These crabs utilize their spatulate chela to gather the soft mud and sandy substrate for extraction of organic matter and specialized setae on their mouth appendages to manipulate sediment particles and to extract food from it. Current study describes the setae of the second maxillipeds within the five Macrophthalmid spp. -

The Species of Hippolyte Leach (Crustacea, Caridea, Hippol Ytidae) from Terminos Lagoon, Southwestern Gulf of Mexico
THE SPECIES OF HIPPOLYTE LEACH (CRUSTACEA, CARIDEA, HIPPOLYTIDAE) FROM TERMINOS LAGOON, SOUTHWESTERN GULF OF MEXICO M.L. Negreiros-Fransozo 1 E. Barba 2 A.J. Sanchez 2 A. Fransozo 1 A. Ráz-Guzmán 2 ABSTRACT. The taxonomic idenlity 01' speci lllcns 01' lhe genus Hippolyte Lcach, 1814 01' Laguna de Términos was considered including the colour and lhe presence absence oftufts ofplumose seta e on the dorsal surl;\ce ofthe. ca rapace and abdomen as secondary characteristics to 1ll0rp h o lo g icall1~at ur e.s oflaxonomic va iu e. Two groups were fo rmed based on appearance: one lransparenl with setae and another green without setae. The analys is of tive morphol ogica l characteri sti cs in adult females of the two groups Illade it possibl e lO identify on ly I-I. =ostericola (Sm ith, 1873). Fecundity and fe rtilily were simil ar (O.5>p>O.2) in both groups. The first zoea ofboth groups were also 111 0rphologicall y sim ilar. Cons iderin g thesc resu lts, iI is concluded that the on ly species co ll ectcd was H. zoslericola an d that it has two phcnotypical types. It is recomended lhat lhe morphological analys is 01' ali larval stages be carried out. KEY WORDS. Hippo~)l t e, zoea, fertility , fccundilY, SW Gu lfofMcxico The crustaceans associated wilh aquati c vegetalion in lhe four g reatesl lagoon systcms in the soulhweslern Gulf 01' Mexico include the carideans among lhe mosl abundanl components (BARBA el aI. 1993). Among these, at leasl one species of the family I-lippolytidac is a dominant component in the two biggest coastal lagoons of this region, Lagun a Madre in Tal11 aul ipas and Laguna de Términos in Campeche (BARBA et aI.