Molecular Data and Biogeography: Resolution of a Controversy Over Evolutionary History of a Pan-Tropical Group of Invertebrates ’
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sydney Harbour: What We Do and Do Not Know About a Highly Diverse Estuary
Marine and Freshwater Research 2015, 66, 1073-1087 © CSIRO 2015 http://dx.doi.org/10.1071/MF15159_AC Supplementary material Sydney Harbour: what we do and do not know about a highly diverse estuary E. L. JohnstonA,B, M. Mayer-PintoA,B, P. A. HutchingsC, E. M. MarzinelliA,B,D, S. T. AhyongC, G. BirchE, D. J. BoothF, R. G. CreeseG, M. A. DoblinH, W. FigueiraI, P. E. GribbenB,D, T. PritchardJ, M. RoughanK, P. D. SteinbergB,D and L. H. HedgeA,B AEvolution and Ecology Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. BSydney Institute of Marine Science, 19 Chowder Bay Road, Mosman, NSW 2088, Australia. CAustralian Museum Research Institute, Australian Museum, 6 College Street, Sydney, NSW 2010, Australia. DCentre for Marine Bio-Innovation, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. ESchool of GeoSciences, The University of Sydney, Sydney, NSW 2006, Australia. FCentre for Environmental Sustainability, School of the Environment, University of Technology, Sydney, NSW 2007, Australia. GNew South Wales Department of Primary Industries, Port Stephens Fisheries Institute, Nelson Bay, NSW 2315, Australia. HPlant Functional Biology and Climate Change Cluster, University of Technology, Sydney, NSW 2007, Australia. ICentre for Research on Ecological Impacts of Coastal Cities, School of Biological Sciences, University of Sydney, NSW 2006, Australia. JWater and Coastal Science Section, New South Wales Office of Environment and Heritage, PO Box A290, Sydney, NSW 1232, Australia. KCoastal and Regional Oceanography Lab, School of Mathematics and Statistics, University of New South Wales, NSW 2052, Australia. -

Archiv Für Naturgeschichte
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Bericht über die Leistuugen in der Carcinologie wälirend des Jahres 1894. Von Dr. F. Hilgendorf und Dr. J. Vosseier*). Verzeichniss der Publicationen. Albert I,, Prince de Monaco: Sur les premieres campagnes de la princesse Alice. Compt. rend. Ac. Sc. Paris T. CXX. — Eine 2 m tief ins Meer eingesenkte Lampe lockt in kurzer Zeit ganze Wolken kleiner Kruster herbei. F. Albrecht, L. K., Ziornow u. a. Primitiae faunae Mosquensis. Congres intern, d'anthrop., arch. et zool. 1892 (Moscou), Materiaux i-eunis etc. 1. partie, Suppl. Nr. 16, 137 S.; Crust. p. 121 — 5. Mos- cou 1893. — 122 Entom. u. 14 Malacostraca, Alcock, A. Natural bist, notes from „Investigator" (Ser. 2) Nr. 1. (continued). (Vergl. Ber. 91, 92, 93 unter Wood-Mason, W.- M. u. Alcock, Alcock). Ann. Mag. (6) XIII p. 225-45, 321—34, 400—411. — Behandelt Deep-sea dredging 1890/91. Spec. Nr. 58 bis 99. Farn. Nematocarcinidae, Honiar., Eryont. (IXyl.), Parapagur., Galath., Inachidae, Cancridae (Platypilumyins)^ Ocypod. (Psopheticus), Leucos. {Ci/monomops), Homolidae. Stomatopoda (2 Sp.), Amphi- poda (l Sp., Farn. Stegoceph., Xyl). 28 neue Sp. od. Variet. Sperma- tozoen V. Munida besclir. p. 324. Stridulationsapp. bei Psophet. Rudim. Augen bei Cymon. u. Andania. Alcock, A. and A. R. Audeison (1). Nat. bist, notes from „Investigator" (2. Ser.) No. 14: An account of a recent coli, of deep sea Crustacea from the Bay of Bengal and Laccadive Sea. Journ. Asiat, soc. of Bengal, Vol. 63 part. IL No. 3. p. 141—185. Tfl. IX. *) Im Allgemeinen sind die Arbeiten über höhere Krebse von Hilgendorf, die über niedere von Vosseier besprochen worden, lieber etwaige Ausnahmen giebt die Unterzeichnung der betreif. -

Download Full Article 1.3MB .Pdf File
Memoirs of the National Museum of Victoria 12 April 1971 Port Phillip Bay Survey 2 https://doi.org/10.24199/j.mmv.1971.32.05 BRACHYURA (CRUSTACEA, DECAPODA) By D. J. G. Griffin and J. C. Yaldwyn* Australian Museum, Sydney Abstract The SurVey C0 Iected 102 specimens of Brachyura *a -| c ! ? belonging to 29 Species and 10 families.m Seven species were taken by the Portland Pier Survey in 1963 five of which are also represented in the Port Phillip Survey collection. Only four of the 38 species known m 3re re resent d the collection. P ? '? The majid Paratymolus talipes and the xanthidTamh-YPilumnuspf acer are recorded from Victoria for the first time; previous records of the graspid\Cyclograpsus audouinii from Victoria are doubtful. Seventeen species known from Port Phillip are not represented in the collection. All are typically cool temperate species well known from SE. Australia. Four species of Pilumnus were represented in the collections and these are compared in detail with other SE. Australian Pilumnus species. Most abundant in Port Phillip are Hahcaranus ovatus and H. rostratus (Hymenosomatidae) Notomithrax minor (Majidae), Ebalia (Phylyxia) intermedia (Leucosiidae), Lilocheira bispinosa (Gone- placidae), Pilumnus tomentosus and P. monilifer (Xanthidae), Nectocardnus integrifrons and Carcinus maenas (Portunidae) and Pinnotheres pisum (Pinnotheridae). The majority of the species are found on the sandy areas around the edge of the Bay, particularly in the W areas; no species was taken in the central deeper parts of the Bay. Ovigerous females of most species were collected in late summer. Parasitism by sacculinas was small and confined to two species of Pilumnus. -

Adaptations by the Locomotor Systems of Terrestrial and Amphibious Crabs Walking Freely on Land and Underwater
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2004 Adaptations by the locomotor systems of terrestrial and amphibious crabs walking freely on land and underwater Jennifer Nuss Schreiner Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Recommended Citation Schreiner, Jennifer Nuss, "Adaptations by the locomotor systems of terrestrial and amphibious crabs walking freely on land and underwater" (2004). LSU Master's Theses. 1349. https://digitalcommons.lsu.edu/gradschool_theses/1349 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. ADAPTATIONS BY THE LOCOMOTOR SYSTEMS OF TERRESTRIAL AND AMPHIBIOUS CRABS WALKING FREELY ON LAND AND UNDERWATER A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in The Department of Biological Sciences by Jennifer Nuss Schreiner B.S., Louisiana State University, 2001 August 2004 ACKNOWLEDGEMENTS I would like to begin by expressing my most heartfelt appreciation to Dr. Jim Belanger. Thank you for giving me the opportunity to be part of your laboratory family, for your everlasting faith in me, and for the endless hours of reassurance when it seemed nothing would ever go as planned. Your patience and generosity mean a great deal to me. -
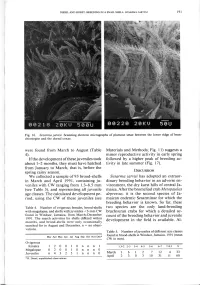
(Table 4). If the Development of These Juveniles Took About 1-2 Months
DIESEL AND HORST: BREEDING IN A SNAIL SHELL: SESARMA JARVISI 191 Fig. 16. Sesarma jarvisi. Scanning electron micrographs of plumose setae between the lower ridge of bran- chiostegite and the dorsal coxae. were found from March to August (Table Materials and Methods; Fig. 11) suggests a 4). minor reproductive activity in early spring If the development of these juveniles took followed by a higher peak of breeding ac- about 1-2 months, they must have hatched tivity in late summer (Fig. 17). from January to March, that is, before the spring rainy season. DISCUSSION We collected a sample of 93 brood-shells Sesarma jarvisi has adopted an extraor- in March and April 1991, containing ju- dinary breeding behavior in an adverse en- veniles with CW ranging from 1.3-8.5 mm vironment, the dry karst hills of central Ja- (see Table 5), and representing all juvenile maica. After the bromeliad crab Metopaulias age classes. The calculated development pe- depressus, it is the second species of Ja- riod, using the CW of these juveniles (see maican endemic Sesarminae for which the breeding behavior is known. So far, these Table 4. Number of ovigerous females, brood-shells two species are the only land-breeding with megalopae, and shells with juveniles <3-mm CW brachyuran crabs for which a detailed ac- found in Windsor, Jamaica, from March-December count of the breeding behavior and juvenile 1991. The search activities for shells differed within months, and brood-shells were only occasionally development in the field is available. Al- searched for in August and December, n = no obser- vations. -

Download at Including Basic Examples on How to Use It
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.11.422154; this version posted January 28, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Title: Measuring Compound Eye Optics with Microscope and MicroCT Images Article Type: Methods Author Names and Affiliations: John Paul Currea1, Yash Sondhi2, Akito Y. Kawahara3, and Jamie Theobald2 1Department of Psychology, Florida International University, Miami, FL 33199, U.S.A. 2Department of Biological Sciences, Florida International University, Miami, FL 33199, U.S.A. 3Florida Museum of Natural History, University of Florida, Gainesville, FL, 32611, U.S.A. Abstract (<200 words): The arthropod compound eye is the most prevalent eye type in the animal kingdom, with an impressive range of shapes and sizes. Studying its natural range of morphologies provides insight into visual ecology, development, and evolution. In contrast to the camera-type eyes we possess, external structures of compound eyes often reveal resolution, sensitivity, and field of view if the eye is spherical. Non-spherical eyes, however, require measuring internal structures using imaging technology like MicroCT (µCT). µCT is a burgeoning 3D X-Ray imaging technique that has been used to image arthropod muscles, brains, ocelli, and eyes. Thus far, there is no efficient tool to automate characterizing compound eye optics in either 2D or 3D data. We present two open-source programs: (1) the ommatidia detecting algorithm (ODA), which automatically measures ommatidia count and diameter in 2D images, and (2) a µCT pipeline (ODA-3D), which calculates anatomical acuity, sensitivity, and field of view across the eye by applying the ODA to 3D data. -

Interactive Segmentation of Crystalline Cones in Compound Eyes
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.15.422850; this version posted December 16, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 InSegtCone: Interactive Segmentation of crystalline Cones in compound 2 eyes 3 4 Abstract 5 6 Understanding the diversity of eyes is crucial to unravel how different animals use vision to 7 interact with their respective environments. To date, comparative studies of eye anatomy are 8 scarce because they often involve time-consuming or inefficient methods. X-ray micro- 9 tomography is a promising high-throughput imaging technique that enables to reconstruct the 10 3D anatomy of eyes, but powerful tools are needed to perform fast conversions of anatomical 11 reconstructions into functional eye models. We developed a computing method named 12 InSegtCone to automatically segment the crystalline cones in the apposition compound eyes of 13 arthropods. Here, we describe the full auto-segmentation process, showcase its application to 14 three different insect compound eyes and evaluate its performance. The auto-segmentation 15 could successfully label the full individual shapes of 60%-80% of the crystalline cones, and is 16 about as accurate and 250 times faster than manual labelling of the individual cones. We 17 believe that InSegtCone can be an important tool for peer scientists to enable extensive 18 comparisons of the diversity of eyes and vision in arthropods. 19 20 Authors and affiliations 21 22 Pierre Tichit1*, Tunhe Zhou2*, Hans Martin Kjer3, Vedrana Andersen Dahl3, Anders Bjorholm 23 Dahl3, and Emily Baird1,4 24 25 ˚Corresponding author at Lund University. -

Behavioural and Molecular Evidence for the Systematic Position of Macrophthalmus (Hemiplax) Hirtipes Hombron & Jacquinot, 18
BEHAVIOURAL AND MOLECULAR EVIDENCE FOR THE SYSTEMATIC POSITION OF MACROPHTHALMUS (HEMIPLAX) HIRTIPES HOMBRON & JACQUINOT, 1846, WITH COMMENTS ON MACROPHTHALMINE SUBGENERA (DECAPODA, BRACHYURA, MACROPHTHALMIDAE) BY COLIN L. MCLAY1,3), JUN KITAURA2) and KEIJI WADA2) 1) School of Biological Sciences, Canterbury University, Christchurch, 8004, New Zealand 2) Department of Biological Science, Faculty of Science, Nara Women’s University, Kitauoya-nishimachi, Nara 630-8506, Japan ABSTRACT The analysis of 16s rRNA data from the New Zealand sentinel crab, Macrophthalmus (Hemiplax) hirtipes (Jacquinot & Hombron, 1846) (Macrophthalminae: Macrophthalmidae) shows that it is the most basal species in the genus Macrophthalmus, and behavioural trait mapping suggests that ancestral behaviours included extended cheliped fighting. Other behaviour found in some sentinel crabs, such as allocleaning and waving displays, were absent in the ancestor and are regarded here as derived traits. It is proposed that the two subgenera Hemiplax and Tasmanoplax be elevated to full generic status. Three subgenera appear to be polyphyletic: Macrophthalmus (26 species), Mareotis (14 species) and Paramareotis (4 species), and to resolve this problem molecular data from the remaining species will need to be added into the analysis. The Australasian fauna contains the four most basal genera of the Macrophthalminae: Tasmanoplax, Hemiplax, Australoplax,andEnigmaplax. Our hypothesis is that these were early off-shoots from Tethyan ancestor(s) that colonized Australasia. RÉSUMÉ L’analyse des données d’ARNr 16S du crabe sentinelle venant de la néo-zélandais, Macroph- thalmus (Hemiplax) hirtipes (Jacquinot et Hombron, 1846) (Macrophthalminae: Macrophthal- midae) montre que cette espèce est la plus basale du genre Macrophthalmus, et l’analyse des traits comportementaux suggère que le comportements de ses les ancêtres incluaient des com- bats avec les chélipédes déployés. -

Number of Ghost Crab Burrows Does Not Correspond to Population Size
Cent. Eur. J. Biol. • 8(9) • 2013 • 843-847 DOI: 10.2478/s11535-013-0208-7 Central European Journal of Biology Number of ghost crab burrows does not correspond to population size Communication Willian T.A.F. Silva1,2,*, Tereza C.S. Calado1 1Integrated Laboratories of Marine and Natural Sciences, Federal University of Alagoas, 57051-090 Maceió-AL, Brazil 2Institute of Evolutionary Sciences of Montpellier, Group of Developmental and Evolutionary Biology, University of Montpellier 2, 34095 Montpellier, France Received 12 December 2012; Accepted 08 May 2013 Abstract: Ghost crabs are distributed worldwide on sandy beaches, and several studies have associated the number of ghost crab burrows with the levels of anthropogenic impacts on the beaches under study. However, our results show that the use of ghost crab Ocypode quadrata burrows to assess levels of anthropogenic impacts on sand beaches may not be accurate, as previously thought, because the number of burrows does not represent an estimate of the population size. In addition, we propose three hypotheses to explain the extremely low number of individuals/number of burrows ratio: the “secret chamber”, the “multiple openings”, and the “one crab, several burrows” hypotheses. We also observed an unusual sex ratio. Keywords: Ocypode quadrata • Ocypodidae • Sandy beaches • Anthropogenic impacts © Versita Sp. z o.o. 1. Introduction [12-15]. In a field survey, we tested the hypothesis that the number of ghost crab O. quadrata burrows on sandy The ghost crab Ocypode quadrata (Fabricius, 1787), beaches corresponds to the estimated number of crabs also known as maria-farinha in Brazil, is the only in the population. -

Territorial Defence in a Network: Audiences Only Matter to Male Fiddler Crabs Primed For
ORE Open Research Exeter TITLE Territorial defense in a network: Audiences only matter to male fiddler crabs primed for confrontation AUTHORS Darden, SK; May, MK; Boyland, NK; et al. JOURNAL Behavioral Ecology DEPOSITED IN ORE 19 June 2019 This version available at http://hdl.handle.net/10871/37589 COPYRIGHT AND REUSE Open Research Exeter makes this work available in accordance with publisher policies. A NOTE ON VERSIONS The version presented here may differ from the published version. If citing, you are advised to consult the published version for pagination, volume/issue and date of publication 1 Territorial defence in a network: audiences only matter to male fiddler crabs primed for 2 confrontation 3 4 Lay Summary: Being part of a social network means that responses to social confrontations 5 are likely to be more complex than they might seem. Indeed, here we find effects of a wider 6 network of conspecifics on an individual’s behaviour in male European fiddler crabs. Males 7 became more aggressive toward intruders if their neighbour was watching when they had 8 previously observed an aggressive interaction between their neighbour and a male territory 9 intruder. 10 11 Abstract 12 13 Territorial contests often occur in the presence of conspecifics not directly involved in the 14 interaction. Actors may alter their behaviour in the presence of this audience, an ‘audience 15 effect’, and audiences themselves may alter their behaviour as a result of observing an 16 interaction, a ‘bystander effect’. Previous work has documented these effects by looking at 17 each in isolation, but to our knowledge, none has investigated their interaction; something 18 that is more likely to represent a realistic scenario for species where individuals aggregate 19 spatially. -

Systematics of the Family Ocypodidae Rafinesque, 1815 (Crustacea: Brachyura), Based on Phylogenetic Relationships, with a Reorga
RAFFLES BULLETIN OF ZOOLOGY 2016 Taxonomy & Systematics RAFFLES BULLETIN OF ZOOLOGY 64: 139–175 Date of publication: 21 July 2016 http://zoobank.org/urn:lsid:zoobank.org:pub:80EBB258-0F6A-4FD6-9886-8AFE317C25F6 Systematics of the family Ocypodidae Rafinesque, 1815 (Crustacea: Brachyura), based on phylogenetic relationships, with a reorganization of subfamily rankings and a review of the taxonomic status of Uca Leach, 1814, sensu lato and its subgenera Hsi-Te Shih1, Peter K. L. Ng2, Peter J. F. Davie3, Christoph D. Schubart4, Michael Türkay5, Reza Naderloo6, Diana Jones7 & Min-Yun Liu8* Abstract. The family Ocypodidae is a group of intertidal brachyuran crabs found in tropical to temperate seas worldwide. While the family has historically included many subfamilies, most of these have now been given separate family status within the superfamily Ocypodoidea. The most recent classification recognises only two subfamilies, the ghost crabs: Ocypodinae Rafinesque, 1815; and the fiddler crabs: Ucinae Dana, 1851. The ghost crabs comprise 21 species in two genera, Ocypode Weber, 1795, and Hoplocypode Sakai & Türkay, 2013. The fiddler crabs are the most species-rich group of this family with 104 species, all belonging to the single genus Uca Leach, 1814, with 12 recognised subgenera. The present study supports 13 groups (= genera) belonging to three revised subfamilies. This result is based on molecular evidence from the nuclear 28S rDNA, and the mitochondrial 16S rDNA and cytochrome oxidase subunit I (COI). The family now also includes the monogeneric Ucididae Števčić, 2005, recently recognised as a separate family for Ucides Rathbun, 1897, herein relegated to a subfamily of the Ocypodidae. -

Spatial Distribution and Substratum
SPATIAL DISTRIBUTION AND SUBSTRATUM PREFERENCE OF THE BRACHYURAN CRAB, MACROPHTHALMUS DEPRESSUS (DECAPODA, OCYPODIDAE) ALONG THE LOWER ESTUARINE MUDFLAT OF MAHI RIVER, GUJARAT, INDIA BY PRANAV J. PANDYA and KAURESH D. VACHHRAJANI1) Division of Environment and Toxicology, Department of Zoology, Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara 390002, Gujarat, India ABSTRACT The distribution of the ocypodid crab, Macrophthalmus depressus Rüppell, 1830, was studied on the lower intertidal mudflats of the Mahi River estuary of the Gulf of Khambhat, Gujarat, India. Zoning of the study area was done based on coastal morphology and sediment composition. Distribution pattern and density were studied using transect and quadrate sampling methods. Five zones with variable sand, silt, and clay composition could be distinguished perpendicular to the shore line. Zones 1 and 2 represented the upper inter tidal area (60% sand), Zones 3 and 4 the mid-inter tidal area (70-80% silt + clay) and Zone 5 the lower inter tidal area (50% sand). Quadrant analysis showed the density of M. depressus burrows as follows: Zone 1, no animals present (hence not further included in the study); Zone 2, 28.66 ± 9.86; Zone 3, 83.20 ± 33.87; Zone 4, 62.00 ± 9.16; and Zone 5, 52.50±24.74 individuals per m2. The mean density of the crab varied from 0 to 83 individuals per linear metre along the transect all over the above mentioned zones. The densier distribution in, and thus presumed preference for, Zones 3 and 4 can be attributed to the sediment composition as well as to the slope, which together make the substrate firm and suitable for easy burrow construction.