Histology of the Human Carotid Sheath Revisited
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neck Dissection Using the Fascial Planes Technique
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY NECK DISSECTION USING THE FASCIAL PLANE TECHNIQUE Patrick J Bradley & Javier Gavilán The importance of identifying the presence larised in the English world in the mid-20th of metastatic neck disease with head and century by Etore Bocca, an Italian otola- neck cancer is recognised as a prominent ryngologist, and his colleagues 5. factor determining patients’ prognosis. The current available techniques to identify Fascial compartments allow the removal disease in the neck all have limitations in of cervical lymphatic tissue by separating terms of accuracy; thus, elective neck dis- and removing the fascial walls of these section is the usual choice for management “containers” along with their contents of the clinically N0 neck (cN0) when the from the underlying vascular, glandular, risk of harbouring occult regional metasta- neural, and muscular structures. sis is significant (≥20%) 1. Methods availa- ble to identify the N+ (cN+) neck include Anatomical basis imaging (CT, MRI, PET), ultrasound- guided fine needle aspiration cytology The basic understanding of fascial planes (USGFNAC), and sentinel node biopsy, in the neck is that there are two distinct and are used depending on resource fascial layers, the superficial cervical fas- availability, for the patient as well as the cia, and the deep cervical fascia (Figures local health service. In many countries, 1A-C). certainly in Africa and Asia, these facilities are not available or affordable. In such Superficial cervical fascia circumstances patients with head and neck cancer whose primary disease is being The superficial cervical fascia is a connec- treated surgically should also have the tive tissue layer lying just below the der- neck treated surgically. -
Anatomical Overview
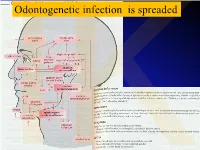
IKOdontogenetic infection is spreaded Možné projevy zlomenin a zánětů IKPossible signs of fractures or inflammations Submandibular space lies between the bellies of the digastric muscles, mandible, mylohyoid muscle and hyoglossus and styloglossus muscles IK IK IK IK IK Submandibulární absces Submandibular abscess IK Sběhlý submandibulární absces Submandibular abscess is getting down IK Submental space lies between the mylohyoid muscles and the investing layer of deep cervical fascia superficially IK IK Spatium peritonsillare IK IK Absces v peritonsilární krajině Abscess in peritonsilar region IK Fasciae Neck fasciae cervicales Demarcate spaces • fasciae – Superficial (investing): • f. nuchae, f. pectoralis, f. deltoidea • invests m. sternocleidomastoideus + trapezius • f. supra/infrahyoidea – pretrachealis (middle neck f.) • form Δ, invests infrahyoid mm. • vagina carotica (carotic sheet) – Prevertebral (deep cervical f.) • Covers scaleni mm. IK• Alar fascia Fascie Fascia cervicalis superficialis cervicales Fascia cervicalis media Fascia cervicalis profunda prevertebralis IKsuperficialis pretrachealis Neck spaces - extent • paravisceral space – Continuation of parafaryngeal space – Nervous and vascular neck bundle • retrovisceral space – Between oesophagus and prevertebral f. – Previsceral space – mezi l. pretrachealis a orgány – v. thyroidea inf./plx. thyroideus impar • Suprasternal space – Between spf. F. and pretracheal one IK– arcus venosus juguli 1 – sp. suprasternale suprasternal Spatia colli 2 – sp. pretracheale pretracheal 3 – -

3 Approach-Related Complications Following Anterior Cervical Spine Surgery: Dysphagia, Dysphonia, and Esophageal Perforations
3 Approach-Related Complications Following Anterior Cervical Spine Surgery: Dysphagia, Dysphonia, and Esophageal Perforations Bharat R. Dave, D. Devanand, and Gautam Zaveri Introduction This chapter analyzes the problems of dysphagia, dysphonia, and esophageal tears during the Pathology involving the anterior subaxial anterior approach to the cervical spine and cervical spine is most commonly accessed suggests ways of prevention and management. through an anterior retropharyngeal approach (Fig. 3.1). While this approach uses tissue planes to access the anterior cervical spine, visceral Dysphagia structures such as the trachea and esophagus and nerves such as the recurrent laryngeal Dysphagia or difficulty in swallowing is a nerve (RLN), superior laryngeal nerve (SLN), and symptom indicative of impairment in the ability pharyngeal plexus are vulnerable to direct or to swallow because of neurologic or structural traction injury (Table 3.1). Complaints such as problems that alter the normal swallowing dysphagia and dysphonia are not rare following process. Postoperative dysphagia is labeled as anterior cervical spine surgery. The treating acute if the patient presents with difficulty in surgeon must be aware of these possible swallowing within 1 week following surgery, complications, must actively look for them in intermediate if the presentation is within 1 to the postoperative period, and deal with them 6 weeks, and chronic if the presentation is longer expeditiously to avoid secondary complications. than 6 weeks after surgery. Common carotid artery Platysma muscle Sternohyoid muscle Vagus nerve Recurrent laryngeal nerve Longus colli muscle Internal jugular artery Anterior scalene muscle Middle scalene muscle External jugular vein Posterior scalene muscle Fig. 3.1 Anterior retropharyngeal approach to the cervical spine. -

Unusual Organization of the Ansa Cervicalis: a Case Report
CASE REPORT ISSN- 0102-9010 UNUSUAL ORGANIZATION OF THE ANSA CERVICALIS: A CASE REPORT Ranjana Verma1, Srijit Das2 and Rajesh Suri3 Department of Anatomy, Maulana Azad Medical College, New Delhi-110002, India. ABSTRACT The superior root of the ansa cervicalis is formed by C1 fibers carried by the hypoglossal nerve, whereas the inferior root is contributed by C2 and C3 nerves. We report a rare finding in a 40-year-old male cadaver in which the vagus nerve fused with the hypoglossal nerve immediately after its exit from the skull on the left side. The vagus nerve supplied branches to the sternohyoid, sternothyroid and superior belly of the omohyoid muscles and also contributed to the formation of the superior root of the ansa cervicalis. In this arrangement, paralysis of the infrahyoid muscles may result following lesion of the vagus nerve anywhere in the neck. The cervical location of the vagus nerve was anterior to the common carotid artery within the carotid sheath. This case report may be of clinical interest to surgeons who perform laryngeal reinnervation and neurologists who diagnose nerve disorders. Key words: Ansa cervicalis, hypoglossal nerve, vagus nerve, variations INTRODUCTION cadaver. The right side was normal. The neck region The ansa cervicalis is a nerve loop formed was dissected and the neural structures in the carotid by the union of superior and inferior roots. The and muscular triangle regions were exposed, with superior root is a branch of the hypoglossal nerve particular attention given to the organization of the containing C1 fibers, whereas the inferior root is ansa cervicalis. -

Head & Neck Surgery Course
Head & Neck Surgery Course Parapharyngeal space: surgical anatomy Dr Pierfrancesco PELLICCIA Pr Benjamin LALLEMANT Service ORL et CMF CHU de Nîmes CH de Arles Introduction • Potential deep neck space • Shaped as an inverted pyramid • Base of the pyramid: skull base • Apex of the pyramid: greater cornu of the hyoid bone Introduction • 2 compartments – Prestyloid – Poststyloid Anatomy: boundaries • Superior: small portion of temporal bone • Inferior: junction of the posterior belly of the digastric and the hyoid bone Anatomy: boundaries Anatomy: boundaries • Posterior: deep fascia and paravertebral muscle • Anterior: pterygomandibular raphe and medial pterygoid muscle fascia Anatomy: boundaries • Medial: pharynx (pharyngobasilar fascia, pharyngeal wall, buccopharyngeal fascia) • Lateral: superficial layer of deep fascia • Medial pterygoid muscle fascia • Mandibular ramus • Retromandibular portion of the deep lobe of the parotid gland • Posterior belly of digastric muscle • 2 ligaments – Sphenomandibular ligament – Stylomandibular ligament Aponeurosis and ligaments Aponeurosis and ligaments • Stylopharyngeal aponeurosis: separates parapharyngeal spaces to two compartments: – Prestyloid – Poststyloid • Cloison sagittale: separates parapharyngeal and retropharyngeal space Aponeurosis and ligaments Stylopharyngeal aponeurosis Muscles stylohyoidien Stylopharyngeal , And styloglossus muscles Prestyloid compartment Contents: – Retromandibular portion of the deep lobe of the parotid gland – Minor or ectopic salivary gland – CN V branch to tensor -

Deep Neck Infections 55
Deep Neck Infections 55 Behrad B. Aynehchi Gady Har-El Deep neck space infections (DNSIs) are a relatively penetrating trauma, surgical instrument trauma, spread infrequent entity in the postpenicillin era. Their occur- from superfi cial infections, necrotic malignant nodes, rence, however, poses considerable challenges in diagnosis mastoiditis with resultant Bezold abscess, and unknown and treatment and they may result in potentially serious causes (3–5). In inner cities, where intravenous drug or even fatal complications in the absence of timely rec- abuse (IVDA) is more common, there is a higher preva- ognition. The advent of antibiotics has led to a continu- lence of infections of the jugular vein and carotid sheath ing evolution in etiology, presentation, clinical course, and from contaminated needles (6–8). The emerging practice antimicrobial resistance patterns. These trends combined of “shotgunning” crack cocaine has been associated with with the complex anatomy of the head and neck under- retropharyngeal abscesses as well (9). These purulent col- score the importance of clinical suspicion and thorough lections from direct inoculation, however, seem to have a diagnostic evaluation. Proper management of a recog- more benign clinical course compared to those spreading nized DNSI begins with securing the airway. Despite recent from infl amed tissue (10). Congenital anomalies includ- advances in imaging and conservative medical manage- ing thyroglossal duct cysts and branchial cleft anomalies ment, surgical drainage remains a mainstay in the treat- must also be considered, particularly in cases where no ment in many cases. apparent source can be readily identifi ed. Regardless of the etiology, infection and infl ammation can spread through- Q1 ETIOLOGY out the various regions via arteries, veins, lymphatics, or direct extension along fascial planes. -

Tentorium Cerebelli: the Bridge Between the Central and Peripheral Nervous System, Part 2
Open Access Review Article DOI: 10.7759/cureus.5679 Tentorium Cerebelli: the Bridge Between the Central and Peripheral Nervous System, Part 2 Bruno Bordoni 1 , Marta Simonelli 2 , Maria Marcella Lagana 3 1. Cardiology, Foundation Don Carlo Gnocchi, Milan, ITA 2. Osteopathy, French-Italian School of Osteopathy, Pisa, ITA 3. Radiology, IRCCS Fondazione Don Carlo Gnocchi Onlus, Milan, ITA Corresponding author: Bruno Bordoni, [email protected] Abstract The tentorium cerebelli is a meningeal portion in relation to the skull, the nervous system, and the cervical tract. In this second part, the article discusses the systematic tentorial relationships, such as the central and cervical neurological connections, the venous circulation and highlights possible clinical alterations that could cause pain. To understand the function of anatomy, we should always remember that every area of the human body is never a segment, but a functional continuum. Categories: Physical Medicine & Rehabilitation, Anatomy, Osteopathic Medicine Keywords: tentorium cerebelli, fascia, pain, venous circulation, neurological connections, cranio Introduction And Background Cervical neurological connections The ansa cervicalis characterizes the first cervical roots and connects all anterior cervical nerve exits with the inferior floor of the oral cavity, the trigeminal system, the respiratory control system, and the sympathetic system. The descending branch of the hypoglossal nerve anastomoses with C1, forming the ansa hypoglossi or ansa cervicalis superior [1]. The inferior root of the ansa cervicalis, also known as descendens cervicalis, is formed by ascendant fibers from spinal nerves C2-C3 and occasionally fibers C4, lying anteriorly to the common carotid artery (it passes laterally or medially to the internal jugular vein upon anatomical variations) [1]. -

Nontraumatic Head and Neck Injuries: a Clinical Approach. Part 2 183
Radiología. 2017;59(3):182---195 www.elsevier.es/rx UPDATE IN RADIOLOGY Nontraumatic head and neck injuries: A clinical ଝ approach. Part 2 ∗ B. Brea Álvarez , L. Esteban García, M. Tunón˜ Gómez, Y. Cepeda Ibarra Hospital Universitario Puerta de Hierro-Majadahonda, Majadahonda, Madrid, Spain Received 3 May 2016; accepted 16 February 2017 KEYWORDS Abstract Nontraumatic emergencies of the head and neck represent a challenge in the field of Emergencies; neuroradiology for two reasons. As explained in the first part of this update, these entities affect Neck injuries; an area where the thorax joins the cranial cavity and can thus compromise both structures; Orbital diseases; second, they are uncommon, so they are not well known. Diseases of the Maintaining the same approach as in the first part, focusing on the clinical presentations in the paranasal sinus; emergency department rather than on the anatomic regions affected, we will study the entities Sialadenitis; that present with two patterns: those that present with a combination of cervical numbness, Cellulitis; dysphagia, and dyspnoea and those that present with acute sensory deficits. In the latter group, Diagnostic imaging; we will specifically focus on visual deficits, because this is the most common symptom that calls Computed for urgent imaging studies. tomography; © 2017 SERAM. Published by Elsevier Espana,˜ S.L.U. All rights reserved. Magnetic resonance imaging PALABRAS CLAVE Urgencias no traumáticas de cabeza y cuello: aproximación desde la clínica. Parte 2 Urgencias; Resumen Las urgencias no traumáticas de cabeza y cuello son un reto en el campo neurorra- Lesiones del cuello; Enfermedades diológico por los motivos referidos en la primera parte: su área de afectación, en la encrucijada orbitarias; del tórax y la cavidad craneal, y su baja incidencia en la urgencia, lo que supone que sean poco conocidas. -

MSS 1. a Patient Presented to a Traumatologist with a Trauma Of
MSS 1. A patient presented to a traumatologist with a trauma of shoulder. What wall of axillary cavity contains foramen trilaterum and foramen quadrilaterum? a) anterior b) posterior c) lateral d) medial e) intermediate 2. A patient presented to a traumatologist with a trauma of leg, which he had sustained at a sport competition. Upon examination, damage of posterior muscle, that is attached to calcaneus by its tendon, was found. This muscle is: a) triceps surae b) tibialis posterior c) popliteus d) fibularis longus e) fibularis brevis 3. In the course of a cesarean section, an incision was made in the pubic area and vagina of rectus abdominis muscle was cut. What does anterior wall of the vagina of rectus abdominis muscle consist of? A. aponeurosis of m. transversus abdominis, m. obliquus internus abdominis. B. aponeurosis of m. transversus abdominis, m. pyramidalis. C. aponeurosis of m. obliquus internus abdominis, m. obliquus externus abdominis. D. aponeurosis of m. transversus abdominis, m. obliquus externus abdominis. E. aponeurosis of m. transversus abdominis, m. obliquus internus abdominis 4. A 30 year-old woman complained of pain in the lower part of her forearm. Traumatologist found that her radio-carpal joint was damaged. This joint is: A. complex, ellipsoid B.simple, ellipsoid C.complex, cylindrical D.simple, cylindrical E.complex condylar 5. A woman was brought by an ambulance to the emergency department with a trauma of the cervical part of her vertebral column. Radiologist diagnosed a fracture of a nonbifid spinous processes of one of her cervical vertebrae. Spinous process of what cervical vertebra is fractured? A.VI. -

An Anomalous Digastric Muscle in the Carotid Sheath: a Case Report with Its
Short Communication 2020 iMedPub Journals Journal of Stem Cell Biology and Transplantation http://journals.imedpub.com Vol. 4 ISS. 4 : sc 37 ISSN : 2575-7725 DOI : 10.21767/2575-7725.4.4.37 8th Edition of International Conference on Clinical and Medical Case Reports - An anomalous digastric muscle in the carotid sheath: a case report with its embryological perspective and clinical relevance Srinivasa Rao Sirasanagandla Sultan Qaboos University, Oman Abstract Key words: Although infrahyoid muscles show considerable variations in Anterior belly, Posterior belly, Variation, Stylohyoid muscle, My- their development, existence of an anomalous digastric muscle lohyoid muscle, Hyoid bone in the neck was seldom reported. During dissection of trian- Anatomy gles of the neck for medical undergraduate students, we came across an anomalous digastric muscle in the carotid sheath of There is a pair of digastric muscles in the neck, and each digas- left side of neck. It was observed in a middle-aged cadaver at tric muscle has the anterior belly and the posterior belly. The College of Medicine and Health Sciences, Sultan Qaboos Uni- anterior belly is attached to the digastric fossa on the base of versity, Muscat, Oman. Digastric muscle was located within the the mandible close to the midline and runs toward the hyoid carotid sheath between the common and internal carotid arter- bone. The posterior belly is attached to the notch of the mas- ies and internal jugular vein. It had two bellies; cranial belly and toid process of the temporal bone and also runs toward the caudal belly which were connected by an intermediate tendon. -

Head and Neck Trauma
Head and Neck Trauma An Interdisciplinary Approach Bearbeitet von Rainer Ottis Seidl, Michael Herzog, Arneborg Ernst 1. Auflage 2006. Buch. 240 S. Hardcover ISBN 978 3 13 140001 7 Format (B x L): 19,5 x 27 cm Weitere Fachgebiete > Medizin > Chirurgie > Orthopädie- und Unfallchirurgie Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. Thieme-Verlag Sommer-Druck Ernst WN 023832/01/01 20.6.2006 Frau Langner Feuchtwangen Head and Neck Trauma TN 140001 Kap-14 14 Diagnosing Injuries of the Larynx and Trachea Flowchart and Checklist Injuries of the Neck, Chap- Treatment of Injuries of the Larynx, Pharynx, Tra- ter 3, p. 25. chea, Esophagus, and Soft Tissues of the Neck, Chapter 23, p. 209. Surgical Anatomy Anteflexion of the head positions the mandible so that it n The laryngeal muscles are divided into those that open the affords effective protection against trauma to the larynx glottis and those that close it. The only muscle that acts to and cervical trachea. Injury to this region occurs if this open the glottis is the posterior cricoarytenoid (posticus) protective reflex function is inhibited and the head is muscle. prevented from bending forward on impact. Spanning the ventral aspect of the cartilage framework, The rigid framework of the larynx is formed by four the cricothyroid muscle is the only laryngeal muscle inner- cartilages: vated by the superior laryngeal nerve. -

Ministry of Education and Science of Ukraine Sumy State University 0
Ministry of Education and Science of Ukraine Sumy State University 0 Ministry of Education and Science of Ukraine Sumy State University SPLANCHNOLOGY, CARDIOVASCULAR AND IMMUNE SYSTEMS STUDY GUIDE Recommended by the Academic Council of Sumy State University Sumy Sumy State University 2016 1 УДК 611.1/.6+612.1+612.017.1](072) ББК 28.863.5я73 С72 Composite authors: V. I. Bumeister, Doctor of Biological Sciences, Professor; L. G. Sulim, Senior Lecturer; O. O. Prykhodko, Candidate of Medical Sciences, Assistant; O. S. Yarmolenko, Candidate of Medical Sciences, Assistant Reviewers: I. L. Kolisnyk – Associate Professor Ph. D., Kharkiv National Medical University; M. V. Pogorelov – Doctor of Medical Sciences, Sumy State University Recommended for publication by Academic Council of Sumy State University as а study guide (minutes № 5 of 10.11.2016) Splanchnology Cardiovascular and Immune Systems : study guide / С72 V. I. Bumeister, L. G. Sulim, O. O. Prykhodko, O. S. Yarmolenko. – Sumy : Sumy State University, 2016. – 253 p. This manual is intended for the students of medical higher educational institutions of IV accreditation level who study Human Anatomy in the English language. Посібник рекомендований для студентів вищих медичних навчальних закладів IV рівня акредитації, які вивчають анатомію людини англійською мовою. УДК 611.1/.6+612.1+612.017.1](072) ББК 28.863.5я73 © Bumeister V. I., Sulim L G., Prykhodko О. O., Yarmolenko O. S., 2016 © Sumy State University, 2016 2 Hippocratic Oath «Ὄμνυμι Ἀπόλλωνα ἰητρὸν, καὶ Ἀσκληπιὸν, καὶ Ὑγείαν, καὶ Πανάκειαν, καὶ θεοὺς πάντας τε καὶ πάσας, ἵστορας ποιεύμενος, ἐπιτελέα ποιήσειν κατὰ δύναμιν καὶ κρίσιν ἐμὴν ὅρκον τόνδε καὶ ξυγγραφὴν τήνδε.