Deep Neck Infections 55
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

7-Khyati Santram
J. Anat. Sciences, 24(2): Dec. 2016, 33-35 Case Report ANATOMICAL VARIATION OF THE TRAPEZIUS MUSCLE- A CASE REPORT Khayati Sant Ram*, Anjali Aggarwal*,Tulika Gupta *,Amandeep kaur*,Jyoti Rajput*, Daisy Sahni* *Department of Anatomy, Post Graduate Institute of Medical Education and Research, Chandigarh, ABSTRACT During routine cadaveric dissection for undergraduate teaching variation in the course and insertion of fibers left trapezius muscle was noticed in two embalmed male cadavers. Some of the occipital fibers while descending towards clavicle got separated from rest of the muscle, inclined medially and inserted on the 1cm area of middle third of superior surface of clavicle. The remaining occipital fibers got inserted after a gap of on 1.5cm on the superior surface of lateral part of clavicle. Detached portion of trapezius muscles was tendinous in insertion. Two structures namely external jugular vein and supraclavicular nerves were seen passing through the gap in one cadaver (Case 1) whereas in other cadaver (Case 2) only external jugular vein was passing through the gap. Knowledge of this variation is clinically important in surgical exploration of posterior triangle.supraclavicular nerve entrapment syndrome and in various approaches involving external jugular vein. Key words: - trapezius muscle, cleidoccipital, supraclavicular nerves INTRODUCTION insertion of clavicular fibers left trapezius muscle of Trapezius muscle(TM) is a flat triangular muscle left side was noticed in two adult male cadavers. which extends over back of the neck and upper Case 1- In 78- years-old male cadaver trapezius thorax. On either side the muscle is attached to muscle of left side took usual origin from the medial medial third of superior nuchal line, external occipital portion of superior nuchal line, external occipital protuberance, ligamentum nuchae and apices of the protuberance, ligamentum nuchae and apices of the spinous processes and their supraspinous ligament spinous processes of thoracic vertebrae. -

Neck Dissection Using the Fascial Planes Technique
OPEN ACCESS ATLAS OF OTOLARYNGOLOGY, HEAD & NECK OPERATIVE SURGERY NECK DISSECTION USING THE FASCIAL PLANE TECHNIQUE Patrick J Bradley & Javier Gavilán The importance of identifying the presence larised in the English world in the mid-20th of metastatic neck disease with head and century by Etore Bocca, an Italian otola- neck cancer is recognised as a prominent ryngologist, and his colleagues 5. factor determining patients’ prognosis. The current available techniques to identify Fascial compartments allow the removal disease in the neck all have limitations in of cervical lymphatic tissue by separating terms of accuracy; thus, elective neck dis- and removing the fascial walls of these section is the usual choice for management “containers” along with their contents of the clinically N0 neck (cN0) when the from the underlying vascular, glandular, risk of harbouring occult regional metasta- neural, and muscular structures. sis is significant (≥20%) 1. Methods availa- ble to identify the N+ (cN+) neck include Anatomical basis imaging (CT, MRI, PET), ultrasound- guided fine needle aspiration cytology The basic understanding of fascial planes (USGFNAC), and sentinel node biopsy, in the neck is that there are two distinct and are used depending on resource fascial layers, the superficial cervical fas- availability, for the patient as well as the cia, and the deep cervical fascia (Figures local health service. In many countries, 1A-C). certainly in Africa and Asia, these facilities are not available or affordable. In such Superficial cervical fascia circumstances patients with head and neck cancer whose primary disease is being The superficial cervical fascia is a connec- treated surgically should also have the tive tissue layer lying just below the der- neck treated surgically. -

Gross Anatomy
www.BookOfLinks.com THE BIG PICTURE GROSS ANATOMY www.BookOfLinks.com Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. The authors and the publisher of this work have checked with sources believed to be reliable in their efforts to provide information that is complete and generally in accord with the standards accepted at the time of publication. However, in view of the possibility of human error or changes in medical sciences, neither the authors nor the publisher nor any other party who has been involved in the preparation or publication of this work warrants that the information contained herein is in every respect accurate or complete, and they disclaim all responsibility for any errors or omissions or for the results obtained from use of the information contained in this work. Readers are encouraged to confirm the infor- mation contained herein with other sources. For example and in particular, readers are advised to check the product information sheet included in the package of each drug they plan to administer to be certain that the information contained in this work is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. This recommendation is of particular importance in connection with new or infrequently used drugs. www.BookOfLinks.com THE BIG PICTURE GROSS ANATOMY David A. Morton, PhD Associate Professor Anatomy Director Department of Neurobiology and Anatomy University of Utah School of Medicine Salt Lake City, Utah K. Bo Foreman, PhD, PT Assistant Professor Anatomy Director University of Utah College of Health Salt Lake City, Utah Kurt H. -

Fossa of Rosenmüller Rosenmüller
Quick Review: Fossa of pharyngeal recess or the fossa of Rosenmüller Rosenmüller. The nasopharynx is a fibromuscular sling suspended from the skull base. The human nasopharynx is mainly derived from the primitive pharynx. It represents the nasal portion of the pharynx behind the nasal cavity and above the free border of the soft palate. The nasopharynx communicates with the nasal cavities through posterior nasal apertures. The choanal orifices along with the posterior edge of the Saggital section of the postnasal space (L E Loh et al 1991) nasal septum form the anterior boundary of the nasopharynx. The The superior constrictor muscle does superior surface of the soft palate not reach the base of skull hence a constitutes its floor and lateral gap (sinus of Morgagni) is velopharyngeal isthum provides created. Fossa of Rosenmüller is a communication between nasopharynx herniation of the nasopharyngeal and oropharynx. The body of mucosa through this deficiency sphenoid, basiocciput and first and between skull base and superior most second cervical vertebrae combine to fibers of the superior constrictor form roof of the nasopharynx. muscle. Through this gap bridged only by the pharyngobasilar fascia, the The part of nasopharynx proximal to eustachian tube enters the the tubal orifice is innervated by the nasopharynx with its two muscles, one maxillary division of the trigeminal (V) on each side. Along the inferior border nerve, and that posterior to the tubal of the two muscles the Fossa of orifice by the glossopharyngeal (IX) Rosenmüller is separated from the nerve. parapharyngeal space by mucosa and pharyngobasilar fascia. Functional studies with contrast and cinefluorography reveal structural The borders of the Fossa of differences between the two Rosenmüller are: components. -
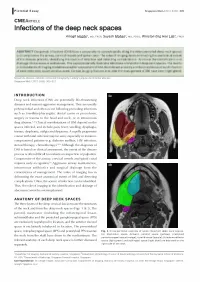
Infections of the Deep Neck Spaces
!Pictorial Essay Singapore Med J 2012; 53(5) 305 CM EARTICLE Infections of the deep neck spaces Amogh Hegdel, MD, FRCR, Suyash Mohan2, MD, PDCC, Winston Eng Hoe Lima, FRCR ABSTRACT Deep neck infections (DNI) have a propensity to spread rapidly along the interconnected deep neck spaces and compromise the airway, cervical vessels and spinal canal. The value of imaging lies in delineating the anatomical extent of the disease process, identifying the source of infection and detecting complications. Its role in the identification and drainage of abscesses is well known. This paper pictorially illustrates infections of important deep neck spaces. The merits and drawbacks of imaging modalities used for assessment of DNI, the relevant anatomy and the possible sources of infection of each deep neck space are discussed. Certain imaging features that alter the management of DNI have been highlighted. Keywords: abscess, cellulitis, computed tomography, Ludwig's angina, peritonsillar abscess Singapore Med J2012; 53(5): 305-312 INTRODUCTION la Deep neck infections (DNI) are potentially life -threatening diseases and warrant aggressive management. They are usually polymicrobial and often occur following preceding infections such as tonsillitis/pharyngitis, dental caries or procedures, surgery or trauma to the head and neck, or in intravenous drug abusers.(1,2) Clinical manifestations of DNI depend on the EJ spaces infected, and include pain, fever, swelling, dysphagia, trismus, dysphonia, otalgia and dyspnoea. A rapidly progressive course with fatal outcome may be seen, especially in immuno- compromised patients (e.g. diabetes mellitus, HIV infection, steroid therapy, chemotherapy).(' -3) Although the diagnosis of DNI is based on clinical assessment, the extent of the disease process is often difficult to evaluate on inspection or palpation. -

Transoral Robotic Assisted Resection of the Parapharyngeal Space
UCLA UCLA Previously Published Works Title Transoral robotic assisted resection of the parapharyngeal space. Permalink https://escholarship.org/uc/item/0wh104s6 Journal Head & neck, 37(2) ISSN 1043-3074 Author Mendelsohn, Abie H Publication Date 2015-02-01 DOI 10.1002/hed.23724 Peer reviewed eScholarship.org Powered by the California Digital Library University of California OPERATIVE TECHNIQUES–PICTORIAL ESSAY Transoral robotic assisted resection of the parapharyngeal space Abie H. Mendelsohn, MD* Director - Robotic Head & Neck Surgery Program, Department of Head and Neck Surgery, David Geffen School of Medicine at University of California – Los Angeles, Los Angeles, California and Jonsson Comprehensive Cancer Center, David Geffen School of Medicine at University of California – Los Angeles, Los Angeles, California. Accepted 28 April 2014 Published online 15 November 2014 in Wiley Online Library (wileyonlinelibrary.com). DOI 10.1002/hed.23724 ABSTRACT: Background. Preliminary case series have reported clinical Results. Transoral robotic assisted resection of a 54- 3 46-mm paraphar- feasibility and safety of a transoral minimally invasive technique to yngeal space mass was performed, utilizing 97 minutes of robotic surgical approach parapharyngeal space masses. With the assistance of the sur- time. Pictorial demonstration of the robotic resection is provided. gical robotic system, tumors within the parapharyngeal space can now Conclusion. Parapharyngeal space tumors have traditionally been be excised safely without neck incisions. A detailed technical description approached via transcervical skin incisions, typically including blunt dissection is included. from tactile feedback. The transoral robotic approach offers magnified 3D Methods. After developing compressive symptoms from a parapharyng- visualization of the parapharyngeal space that allows for complete and safe eal space lipomatous tumor, the patient was referred by his primary oto- resection. -

Unusual Organization of the Ansa Cervicalis: a Case Report
CASE REPORT ISSN- 0102-9010 UNUSUAL ORGANIZATION OF THE ANSA CERVICALIS: A CASE REPORT Ranjana Verma1, Srijit Das2 and Rajesh Suri3 Department of Anatomy, Maulana Azad Medical College, New Delhi-110002, India. ABSTRACT The superior root of the ansa cervicalis is formed by C1 fibers carried by the hypoglossal nerve, whereas the inferior root is contributed by C2 and C3 nerves. We report a rare finding in a 40-year-old male cadaver in which the vagus nerve fused with the hypoglossal nerve immediately after its exit from the skull on the left side. The vagus nerve supplied branches to the sternohyoid, sternothyroid and superior belly of the omohyoid muscles and also contributed to the formation of the superior root of the ansa cervicalis. In this arrangement, paralysis of the infrahyoid muscles may result following lesion of the vagus nerve anywhere in the neck. The cervical location of the vagus nerve was anterior to the common carotid artery within the carotid sheath. This case report may be of clinical interest to surgeons who perform laryngeal reinnervation and neurologists who diagnose nerve disorders. Key words: Ansa cervicalis, hypoglossal nerve, vagus nerve, variations INTRODUCTION cadaver. The right side was normal. The neck region The ansa cervicalis is a nerve loop formed was dissected and the neural structures in the carotid by the union of superior and inferior roots. The and muscular triangle regions were exposed, with superior root is a branch of the hypoglossal nerve particular attention given to the organization of the containing C1 fibers, whereas the inferior root is ansa cervicalis. -

Head & Neck Surgery Course
Head & Neck Surgery Course Parapharyngeal space: surgical anatomy Dr Pierfrancesco PELLICCIA Pr Benjamin LALLEMANT Service ORL et CMF CHU de Nîmes CH de Arles Introduction • Potential deep neck space • Shaped as an inverted pyramid • Base of the pyramid: skull base • Apex of the pyramid: greater cornu of the hyoid bone Introduction • 2 compartments – Prestyloid – Poststyloid Anatomy: boundaries • Superior: small portion of temporal bone • Inferior: junction of the posterior belly of the digastric and the hyoid bone Anatomy: boundaries Anatomy: boundaries • Posterior: deep fascia and paravertebral muscle • Anterior: pterygomandibular raphe and medial pterygoid muscle fascia Anatomy: boundaries • Medial: pharynx (pharyngobasilar fascia, pharyngeal wall, buccopharyngeal fascia) • Lateral: superficial layer of deep fascia • Medial pterygoid muscle fascia • Mandibular ramus • Retromandibular portion of the deep lobe of the parotid gland • Posterior belly of digastric muscle • 2 ligaments – Sphenomandibular ligament – Stylomandibular ligament Aponeurosis and ligaments Aponeurosis and ligaments • Stylopharyngeal aponeurosis: separates parapharyngeal spaces to two compartments: – Prestyloid – Poststyloid • Cloison sagittale: separates parapharyngeal and retropharyngeal space Aponeurosis and ligaments Stylopharyngeal aponeurosis Muscles stylohyoidien Stylopharyngeal , And styloglossus muscles Prestyloid compartment Contents: – Retromandibular portion of the deep lobe of the parotid gland – Minor or ectopic salivary gland – CN V branch to tensor -

Nontraumatic Head and Neck Injuries: a Clinical Approach. Part 2 183
Radiología. 2017;59(3):182---195 www.elsevier.es/rx UPDATE IN RADIOLOGY Nontraumatic head and neck injuries: A clinical ଝ approach. Part 2 ∗ B. Brea Álvarez , L. Esteban García, M. Tunón˜ Gómez, Y. Cepeda Ibarra Hospital Universitario Puerta de Hierro-Majadahonda, Majadahonda, Madrid, Spain Received 3 May 2016; accepted 16 February 2017 KEYWORDS Abstract Nontraumatic emergencies of the head and neck represent a challenge in the field of Emergencies; neuroradiology for two reasons. As explained in the first part of this update, these entities affect Neck injuries; an area where the thorax joins the cranial cavity and can thus compromise both structures; Orbital diseases; second, they are uncommon, so they are not well known. Diseases of the Maintaining the same approach as in the first part, focusing on the clinical presentations in the paranasal sinus; emergency department rather than on the anatomic regions affected, we will study the entities Sialadenitis; that present with two patterns: those that present with a combination of cervical numbness, Cellulitis; dysphagia, and dyspnoea and those that present with acute sensory deficits. In the latter group, Diagnostic imaging; we will specifically focus on visual deficits, because this is the most common symptom that calls Computed for urgent imaging studies. tomography; © 2017 SERAM. Published by Elsevier Espana,˜ S.L.U. All rights reserved. Magnetic resonance imaging PALABRAS CLAVE Urgencias no traumáticas de cabeza y cuello: aproximación desde la clínica. Parte 2 Urgencias; Resumen Las urgencias no traumáticas de cabeza y cuello son un reto en el campo neurorra- Lesiones del cuello; Enfermedades diológico por los motivos referidos en la primera parte: su área de afectación, en la encrucijada orbitarias; del tórax y la cavidad craneal, y su baja incidencia en la urgencia, lo que supone que sean poco conocidas. -

6. the Pharynx the Pharynx, Which Forms the Upper Part of the Digestive Tract, Consists of Three Parts: the Nasopharynx, the Oropharynx and the Laryngopharynx
6. The Pharynx The pharynx, which forms the upper part of the digestive tract, consists of three parts: the nasopharynx, the oropharynx and the laryngopharynx. The principle object of this dissection is to observe the pharyngeal constrictors that form the back wall of the vocal tract. Because the cadaver is lying face down, we will consider these muscles from the back. Figure 6.1 shows their location. stylopharyngeus suuperior phayngeal constrictor mandible medial hyoid bone phayngeal constrictor inferior phayngeal constrictor Figure 6.1. Posterior view of the muscles of the pharynx. Each of the three pharyngeal constrictors has a left and right part that interdigitate (join in fingerlike branches) in the midline, forming a raphe, or union. This raphe forms the back wall of the pharynx. The superior pharyngeal constrictor is largely in the nasopharynx. It has several origins (some texts regard it as more than one muscle) one of which is the medial pterygoid plate. It assists in the constriction of the nasopharynx, but has little role in speech production other than helping form a site against which the velum may be pulled when forming a velic closure. The medial pharyngeal constrictor, which originates on the greater horn of the hyoid bone, also has little function in speech. To some extent it can be considered as an elevator of the hyoid bone, but its most important role for speech is simply as the back wall of the vocal tract. The inferior pharyngeal constrictor also performs this function, but plays a more important role constricting the pharynx in the formation of pharyngeal consonants. -

Esophagopharyngeal Perforation and Prevertebral Abscess After Anterior Cervical Discectomy and Fusion: a Case Report
232 Case Report Esophagopharyngeal perforation and prevertebral abscess after anterior cervical discectomy and fusion: a case report Jay K. Shah1^, Filippo Romanelli1, Jason Yang2, Naina Rao3, Michael C. Gerling4 1Division of Orthopedic Surgery, Department of Orthopedic Surgery, Jersey City Medical Center – Robertwood Johnson Barnabas Health, Jersey City, NJ, USA; 2Robert Wood Johnson University Hospital, RWJBarnabas Health, New Brunswick, NJ, USA; 3New York Grossman School of Medicine, NYU Langone Health, New York, NY, USA; 4Chief of Spine Surgery, Department of Orthopaedic Surgery, New York University Langone Hospital-Brooklyn, Tribeca, New York, NY, USA Correspondence to: Jay K. Shah, DO. Division of Orthopedic Surgery, Department of Orthopedic Surgery, Jersey City Medical Center – Robertwood Johnson Barnabas Health, 355 Grand Street, Jersey City, NJ 07302, USA. Email: [email protected]. Abstract: Anterior cervical discectomy and fusion (ACDF) represents one of the most commonly performed spine surgeries. Dysphagia secondary to esophageal injury during retraction is one of the most common complications, and usually leads to self-limiting dysphagia. However, actual perforation and violation of the esophageal tissue is much rarer and can lead to delayed deep infections. Prevertebral abscess’ are one of the most feared complications after ACDF, as they can lead to severe tissue swelling, osteomyelitis, hardware failure, and even death. Due to their rarity, a gold standard of workup and treatment is still unknown. A healthy 47-year-old female presents 9 months after a C4–C7 ACDF done at an outside institution with a large prevertebral abscess, osteomyelitis, hardware failure, and pseudoarthrosis secondary to esophagopharyngeal defect and prominent hardware. Overall, the patient underwent eight surgeries, and required an extended course of intravenous (IV) antibiotics, multiple diagnostic procedures, and complex soft tissue coverage using an anterolateral thigh free flap. -

An Anomalous Digastric Muscle in the Carotid Sheath: a Case Report with Its
Short Communication 2020 iMedPub Journals Journal of Stem Cell Biology and Transplantation http://journals.imedpub.com Vol. 4 ISS. 4 : sc 37 ISSN : 2575-7725 DOI : 10.21767/2575-7725.4.4.37 8th Edition of International Conference on Clinical and Medical Case Reports - An anomalous digastric muscle in the carotid sheath: a case report with its embryological perspective and clinical relevance Srinivasa Rao Sirasanagandla Sultan Qaboos University, Oman Abstract Key words: Although infrahyoid muscles show considerable variations in Anterior belly, Posterior belly, Variation, Stylohyoid muscle, My- their development, existence of an anomalous digastric muscle lohyoid muscle, Hyoid bone in the neck was seldom reported. During dissection of trian- Anatomy gles of the neck for medical undergraduate students, we came across an anomalous digastric muscle in the carotid sheath of There is a pair of digastric muscles in the neck, and each digas- left side of neck. It was observed in a middle-aged cadaver at tric muscle has the anterior belly and the posterior belly. The College of Medicine and Health Sciences, Sultan Qaboos Uni- anterior belly is attached to the digastric fossa on the base of versity, Muscat, Oman. Digastric muscle was located within the the mandible close to the midline and runs toward the hyoid carotid sheath between the common and internal carotid arter- bone. The posterior belly is attached to the notch of the mas- ies and internal jugular vein. It had two bellies; cranial belly and toid process of the temporal bone and also runs toward the caudal belly which were connected by an intermediate tendon.