Cellular Angiofibroma in the Space of Retzius: a Case Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
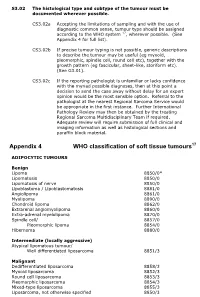
Appendix 4 WHO Classification of Soft Tissue Tumours17
S3.02 The histological type and subtype of the tumour must be documented wherever possible. CS3.02a Accepting the limitations of sampling and with the use of diagnostic common sense, tumour type should be assigned according to the WHO system 17, wherever possible. (See Appendix 4 for full list). CS3.02b If precise tumour typing is not possible, generic descriptions to describe the tumour may be useful (eg myxoid, pleomorphic, spindle cell, round cell etc), together with the growth pattern (eg fascicular, sheet-like, storiform etc). (See G3.01). CS3.02c If the reporting pathologist is unfamiliar or lacks confidence with the myriad possible diagnoses, then at this point a decision to send the case away without delay for an expert opinion would be the most sensible option. Referral to the pathologist at the nearest Regional Sarcoma Service would be appropriate in the first instance. Further International Pathology Review may then be obtained by the treating Regional Sarcoma Multidisciplinary Team if required. Adequate review will require submission of full clinical and imaging information as well as histological sections and paraffin block material. Appendix 4 WHO classification of soft tissue tumours17 ADIPOCYTIC TUMOURS Benign Lipoma 8850/0* Lipomatosis 8850/0 Lipomatosis of nerve 8850/0 Lipoblastoma / Lipoblastomatosis 8881/0 Angiolipoma 8861/0 Myolipoma 8890/0 Chondroid lipoma 8862/0 Extrarenal angiomyolipoma 8860/0 Extra-adrenal myelolipoma 8870/0 Spindle cell/ 8857/0 Pleomorphic lipoma 8854/0 Hibernoma 8880/0 Intermediate (locally -

Surgical Approaches to Fractures of the Acetabulum and Pelvis Joel M
Surgical Approaches to Fractures of the Acetabulum and Pelvis Joel M. Matta, M.D. Sponsored by Mizuho OSI APPROACHES TO THE The table will also stably position the ACETABULUM limb in a number of different positions. No one surgical approach is applicable for all acetabulum fractures. KOCHER-LANGENBECK After examination of the plain films as well as the CT scan the surgeon should APPROACH be knowledgeable of the precise anatomy of the fracture he or she is The Kocher-Langenbeck approach is dealing with. A surgical approach will primarily an approach to the posterior be selected with the expectation that column of the Acetabulum. There is the entire reduction and fixation can excellent exposure of the be performed through the surgical retroacetabular surface from the approach. A precise knowledge of the ischial tuberosity to the inferior portion capabilities of each surgical approach of the iliac wing. The quadrilateral is also necessary. In order to maximize surface is accessible by palpation the capabilities of each surgical through the greater or lesser sciatic approach it is advantageous to operate notch. A less effective though often the patient on the PROfx® Pelvic very useful approach to the anterior Reconstruction Orthopedic Fracture column is available by manipulation Table which can apply traction in a through the greater sciatic notch or by distal and/or lateral direction during intra-articular manipulation through the operation. the Acetabulum (Figure 1). Figure 2. Fractures operated through the Kocher-Langenbeck approach. Figure 3. Positioning of the patient on the PROfx® surgical table for operations through the Kocher-Lagenbeck approach. -

The Role of Cytogenetics and Molecular Diagnostics in the Diagnosis of Soft-Tissue Tumors Julia a Bridge
Modern Pathology (2014) 27, S80–S97 S80 & 2014 USCAP, Inc All rights reserved 0893-3952/14 $32.00 The role of cytogenetics and molecular diagnostics in the diagnosis of soft-tissue tumors Julia A Bridge Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha, NE, USA Soft-tissue sarcomas are rare, comprising o1% of all cancer diagnoses. Yet the diversity of histological subtypes is impressive with 4100 benign and malignant soft-tissue tumor entities defined. Not infrequently, these neoplasms exhibit overlapping clinicopathologic features posing significant challenges in rendering a definitive diagnosis and optimal therapy. Advances in cytogenetic and molecular science have led to the discovery of genetic events in soft- tissue tumors that have not only enriched our understanding of the underlying biology of these neoplasms but have also proven to be powerful diagnostic adjuncts and/or indicators of molecular targeted therapy. In particular, many soft-tissue tumors are characterized by recurrent chromosomal rearrangements that produce specific gene fusions. For pathologists, identification of these fusions as well as other characteristic mutational alterations aids in precise subclassification. This review will address known recurrent or tumor-specific genetic events in soft-tissue tumors and discuss the molecular approaches commonly used in clinical practice to identify them. Emphasis is placed on the role of molecular pathology in the management of soft-tissue tumors. Familiarity with these genetic events -

Aggressive Angiomyxoma in Pregnancy: a Rare and Commonly Misdiagnosed Entity Kamal Malukani, Amit V
Published online: 2020-02-19 Case Report Access this article online Quick Response Code: Aggressive angiomyxoma in pregnancy: A rare and commonly misdiagnosed entity Kamal Malukani, Amit V. Varma, Devashish Choudhary, Shilpi Dosi Website: www.jlponline.org Abstract: DOI: 10.4103/JLP.JLP_179_17 Aggressive angiomyxoma (AAM) is an uncommon mesenchymal tumor that predominantly involves the pelvis and perineum of young females. It is often clinically mistaken for more common superficial lesions such as vaginal cysts, labial cysts, and lipomas. A review of the medical literature reveals very few cases of AAM reported in pregnancy. We describe a rare case of AAM in pregnancy, clinically misdiagnosed as prolapsed cervical fibroid. Key words: Aggressive angiomyxoma, mesenchymal tumors, pregnancy Introduction (G2 P1 L1), married for 5 years, presented with bleeding per vagina for 3 days. She ggressive angiomyxoma (AAM) is also complained of huge mass coming out Aa rare mesenchymal neoplasm of of vagina, difficulty in walking, and pain vulvo‑perineal region and usually found in abdomen and lower back. There was [1] in women of reproductive age group. It no history of fever, weight loss, trauma, is a slow‑growing, low‑grade neoplasm bowel or bladder disturbance, and use of but is locally infiltrative, and relapse any contraceptive. Her menstrual history has been reported in about 30%–40% of and past history were unremarkable. Local cases even after many years of complete examination showed a large pedunculated, resection.[2] Rarely, AAM can metastasize well‑circumscribed, ulcerated mass coming to lungs, peritoneum, and lymph nodes.[3] out of the vagina. Only a few cases of AAM coexistent Ultrasound of abdomen showed with pregnancy are reported in medical bulky anteverted uterus, measuring literature.[4,5] AAM grows to a huge size 9.6 cm × 7.6 cm × 5.4 cm. -

Desterrando El Término Ewing-Like Sarcoma: ¿Qué Entidades Se Cobijaban Bajo Esa Denominación?
Desterrando el término Ewing-like sarcoma: ¿Qué entidades se cobijaban bajo esa denominación? Sílvia Bagué Servei de Patologia Hospital de la Santa Creu i Sant Pau Universitat Autònoma de Barcelona #SEOM20 No disclosures Small round cell sarcomas (SRCS) - Group of malignant neoplasms sharing histological similar features - Mainly in children & young adults - High-grade by definition. Often “translocated-sarcomas” • Ewing sarcoma • “Ewing-like” sarcomas • Desmoplastic SRCT • Alveolar rhabdomyosarcoma • Poorly diff sinovial sarcoma • Mesenchymal chondrosarcoma • High-grade myxoid liposarcoma • Small cell osteosarcoma • Undiff sarcoma with round cell morphology (USRCS) #SEOM203 Ewing sarcoma • Most common round cell sarcoma • 1st - 2nd decade • 85% diaphysis-metaphysis long bones > pelvis, ribs, chest wall (‘Askin’) • 15% extraeskeletal (adults) • Key morphologic features: uniform monotonous round cells, fine chromatin, inconspicious nucleoli, scant clear to pale cytoplasm • Key immunohistochemical stains: strong and diffuse membranous CD99 expression; NKX2+, PAX7+ • Genetics: fusion between EFT genes (FUS, EWSR1) and TAF15 genes and ETS transcription factor family members (Fli-1, ERG, ETV1, ETV4, FEV, E1AF, ZSG) 85-90% t(11;22)(q24;q12) EWSR1- FLI1 fusion gene 5-10 % t(21;22)(q22;q12) EWSR1-ERG fusion gene CD99 FISH EWSR1 #SEOM204 Desmoplastic small round cell tumor Clinical history Boy, 12 y-o. Intraabdominal mass #SEOM205 DSRCT: IHC & genetics Malignant round cell tumor with desmoplastic stroma, poliphenotypic differentiation and translocation -

The 2020 WHO Classification of Soft Tissue Tumours: News and Perspectives
PATHOLOGICA 2021;113:70-84; DOI: 10.32074/1591-951X-213 Review The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives Marta Sbaraglia1, Elena Bellan1, Angelo P. Dei Tos1,2 1 Department of Pathology, Azienda Ospedale Università Padova, Padova, Italy; 2 Department of Medicine, University of Padua School of Medicine, Padua, Italy Summary Mesenchymal tumours represent one of the most challenging field of diagnostic pathol- ogy and refinement of classification schemes plays a key role in improving the quality of pathologic diagnosis and, as a consequence, of therapeutic options. The recent publica- tion of the new WHO classification of Soft Tissue Tumours and Bone represents a major step toward improved standardization of diagnosis. Importantly, the 2020 WHO classi- fication has been opened to expert clinicians that have further contributed to underline the key value of pathologic diagnosis as a rationale for proper treatment. Several rel- evant advances have been introduced. In the attempt to improve the prediction of clinical behaviour of solitary fibrous tumour, a risk assessment scheme has been implemented. NTRK-rearranged soft tissue tumours are now listed as an “emerging entity” also in con- sideration of the recent therapeutic developments in terms of NTRK inhibition. This deci- sion has been source of a passionate debate regarding the definition of “tumour entity” as well as the consequences of a “pathology agnostic” approach to precision oncology. In consideration of their distinct clinicopathologic features, undifferentiated round cell sarcomas are now kept separate from Ewing sarcoma and subclassified, according to the underlying gene rearrangements, into three main subgroups (CIC, BCLR and not Received: October 14, 2020 ETS fused sarcomas) Importantly, In order to avoid potential confusion, tumour entities Accepted: October 19, 2020 such as gastrointestinal stroma tumours are addressed homogenously across the dif- Published online: November 3, 2020 ferent WHO fascicles. -

Axial CT Cystogram
81 y/o male with abdominal pain s/p cystoscopy Edward Gillis, DO David Karimeddini, MD Axial CT Cystogram Axial CT Cystogram Axial CT Cystogram Coronal CT Cystogram ? Intra- and extraperitoneal bladder rupture Axial CT Cystogram: At the mid pelvis, contrast is seen extravasating into the left paracolic gutter, indicating an intraperitoneal component. Axial CT Cystogram: Lower down, perivesical contrast is seen. Foley catheter is present within the bladder. Axial CT Cystogram: Contrast extravasation localized around the right ureterovesical junction. Coronal CT Cystogram: Perivesical contrast around the dome of the bladder (blue arrow). Contrast is also demonstrated in the left paracolic gutter, indicating an intraperitoneal component (green arrow). Sagittal CT Cystogram: Defect in the bladder dome (yellow arrow) with perivesical contrast. Bladder Rupture Imaging Features • Intraperitoneal rupture – Contrast extravasates into the paracolic gutters and outlines loops of bowel. – Layering of contrast in dependent areas (Pouch of Douglas, Morrison’s Pouch) – Look for bladder dome defect • Extraperitoneal Rupture – Extravasation into extraperitoneal spaces, most commonly the retropubic space of Retzius – May see contrast extravasation into the anterior abdominal wall, thigh, and scrotum Bladder Rupture General Features • Extraperitoneal – 62% of all bladder ruptures – Usually secondary to pelvic fracture; fragment lacerates the base of the bladder. – Treatment is usually medical management with Abx and catheterization • Intraperitoneal – 25% of bladder ruptures – Trauma to abdomen with full bladder – May mimic acute renal failure – Treatment requires surgery to repair bladder dome • Combined – 12% of ruptures – Findings of both intraperitoneal and extraperitoneal ruptures References 1. Brant, W. E., & Helms, C. A. (2012). Fundamentals of diagnostic radiology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins 2. -

Anatomy of Pelvic Floor Dysfunction
Anatomy of Pelvic Floor Dysfunction Marlene M. Corton, MD KEYWORDS Pelvic floor Levator ani muscles Pelvic connective tissue Ureter Retropubic space Prevesical space NORMAL PELVIC ORGAN SUPPORT The main support of the uterus and vagina is provided by the interaction between the levator ani (LA) muscles (Fig. 1) and the connective tissue that attaches the cervix and vagina to the pelvic walls (Fig. 2).1 The relative contribution of the connective tissue and levator ani muscles to the normal support anatomy has been the subject of controversy for more than a century.2–5 Consequently, many inconsistencies in termi- nology are found in the literature describing pelvic floor muscles and connective tissue. The information presented in this article is based on a current review of the literature. LEVATOR ANI MUSCLE SUPPORT The LA muscles are the most important muscles in the pelvic floor and represent a crit- ical component of pelvic organ support (see Fig. 1). The normal levators maintain a constant state of contraction, thus providing an active floor that supports the weight of the abdominopelvic contents against the forces of intra-abdominal pressure.6 This action is thought to prevent constant or excessive strain on the pelvic ‘‘ligaments’’ and ‘‘fascia’’ (Fig. 3A). The normal resting contraction of the levators is maintained by the action of type I (slow twitch) fibers, which predominate in this muscle.7 This baseline activity of the levators keeps the urogenital hiatus (UGH) closed and draws the distal parts of the urethra, vagina, and rectum toward the pubic bones. Type II (fast twitch) muscle fibers allow for reflex muscle contraction elicited by sudden increases in abdominal pressure (Fig. -

Pelvis + Perineum Pelvic Cavity
Pelvis + Perineum Pelvic Cavity Enclosed by bony, ligamentous and muscular wall Contains the urinary bladder, ureters, pelvic genital organs, rectum, blood vessels, lymphatics and nerves Pelvic inlet (superior pelvic aperture) Pelvic outlet (inferior pelvic aperture) Superior Apeture Inferior Pelvic Border Lesser (True) Pelvis (pelvis minor) Location of pelvic viscera – the urinary bladder and reproductive organs such as the uterus and ovaries Bounded by the hip bones, sacrum, and coccyx Limited inferiorly by the musculofascial pelvic diaphragm Pelvic Walls and Floors Anterior pelvic wall – is formed primarily by the bodies and rami of the pubic bones and the pubic symphysis Lateral pelvic walls – formed by the hip bones and the obturator internus muscles Anterior Pelvic Wall Pelvic Walls and Floor Posterior Pelvic Wall – formed by the sacrum and coccyx, adjacent parts of the ilia, and the S-I joints; piriformis muscle covers the area Posterior Pelvic Wall Pelvic Floor Formed by the funnel shaped pelvic diaphragm – consists of the levator ani and coccygeus muscles and their fascia Stretches between the pubis anteriorly and the coccyx posteriorly and from one lateral pelvic wall to the other Levator Ani Three parts – the pubococcygeus, the puborectalis and the iliococcygeus. Collectively they run from the body of the pubis, the tendinous arch of the obturator fascia and the ischial spine TO the perineal body, the coccyx, the anococcygeal ligament, the walls of the prostate or vagina, the rectum and the anal canal Innervated -

MR Imaging of Aggressive Angiomyxoma of the Female Pelvis: Case Report
MR Imaging of Aggressive Angiomyxoma of the Female Pelvis: Case Report Hye Mi Kim1, Ji Eun Pyo2, Nam Hoon Cho2, Nam Kyu Kim3, and Myeong-Jin Kim1 Aggressive angiomyxoma is a rare tumor that predominantly occurs in the female genital tract. Because of its high tendency for local recurrence, preoperative diagnosis is important to ensure wide excision and reduce recurrence risk. But it is often misdiagnosed due to its rarity. We present two cases of aggressive angiomyxoma: a recurrent case that was initially misdiagnosed and a preoperatively diagnosed case. Index words : Myxoma Magnetic resonance (MR) Vulva Female Perineum Case 1 Introduction A 43-year-old woman presented with a non tender mass in the right perirectal area in 1999. Magnetic Aggressive angiomyxoma is a recently characterized resonance (MR) imaging showed a large soft-tissue, 12 benign mesenchymal tumor occurring predominantly ×8×5 cm mass, mainly located at the right perineum in the perineal area, with a strong female extending to the perirectal space. The mass displaced predominance in the 3rd-5th decades of life (1). It has a the adjacent soft tissue and splitted the internal and high propensity for local recurrence, thus preoperative external right levator ani sphincter muscle. It was diagnosis is important for planning surgical isointense to muscle on T1-wighted and hyperintense management (2). But it has been often misdiagnosed on T2-weighted images, with a whirling pattern (Fig. 1). due to its rarity (3). Differential diagnosis included a neurogenic tumor, To our knowledge, the imagings of aggressive myxoid liposarcoma, angiomyolipoma or other soft angiomyxoma have not been reported in Korea. -

Cellular Angiofibroma: a Rare Vulvar Neoplasm Distinct from Aggressive Angiomyxoma Jasvinder Kaur Bhatia*1, R Nangia2
Case Report Cellular Angiofibroma: A Rare Vulvar Neoplasm Distinct From Aggressive Angiomyxoma Jasvinder Kaur Bhatia*1, R Nangia2 1Department of Pathology, Armed Forces Medical college Pune, India 2SR Adv Pathology, CHCC Lucknow, India Keywords: Cellular Angiofibroma, Vulva, Immunohistochemistry, Aggressive Angiomyxoma. ABSTRACT Cellular angiofibroma (CA) is a rare benign mesenchymal neoplasm of the genital region of both the genders. In women, it arises in late reproductive age and can be cured by complete local excision. It is characterized by a bland spindle cell population and numerous small- to medium-sized vessels with hyalinization. We report a rare case in a postmenopausal woman with a view to highlight the importance of differentiating it from similar more aggressive tumours like aggressive angiomyxoma. A 56 year old woman presented with superficial painless swelling of the vulva and the mass was surgically excised. Histological examination revealed spindle cell proliferation and hyalinised blood vessels in a loosely cellular stroma. Immunohistochemistry revealed positivity for vimentin and CD34 and positivity for ER & PR. *Corresponding author: Dr Jasvinder Kaur Bhatia, Department of Pathology, Armed Forces Medical College, Pune-40, India Phone: +91 - 8552825142 E-mail: [email protected] This work is licensed under the Creative Commons Attribution 4.0 License. Published by Pacific Group of e-Journals (PaGe) C-214 Cellular Angiofibroma Introduction Immunohistochemistry: The tumour cells were positive Cellular Angiofibroma (CA) is a rare benign mesenchymal for vimentin and CD34, ER and PR. (Fig 4, 5, 6, 7) The neoplasm of the genital region of both the genders. It arises tumour was negative for S-100 protein, actin, desmin and in women of late reproductive age [1, 2] and can be cured EMA. -
Conversion of Morphology of ICD-O-2 to ICD-O-3
NATIONAL INSTITUTES OF HEALTH National Cancer Institute to Neoplasms CONVERSION of NEOPLASMS BY TOPOGRAPHY AND MORPHOLOGY from the INTERNATIONAL CLASSIFICATION OF DISEASES FOR ONCOLOGY, SECOND EDITION to INTERNATIONAL CLASSIFICATION OF DISEASES FOR ONCOLOGY, THIRD EDITION Edited by: Constance Percy, April Fritz and Lynn Ries Cancer Statistics Branch, Division of Cancer Control and Population Sciences Surveillance, Epidemiology and End Results Program National Cancer Institute Effective for cases diagnosed on or after January 1, 2001 TABLE OF CONTENTS Introduction .......................................... 1 Morphology Table ..................................... 7 INTRODUCTION The International Classification of Diseases for Oncology, Third Edition1 (ICD-O-3) was published by the World Health Organization (WHO) in 2000 and is to be used for coding neoplasms diagnosed on or after January 1, 2001 in the United States. This is a complete revision of the Second Edition of the International Classification of Diseases for Oncology2 (ICD-O-2), which was used between 1992 and 2000. The topography section is based on the Neoplasm chapter of the current revision of the International Classification of Diseases (ICD), Tenth Revision, just as the ICD-O-2 topography was. There is no change in this Topography section. The morphology section of ICD-O-3 has been updated to include contemporary terminology. For example, the non-Hodgkin lymphoma section is now based on the World Health Organization Classification of Hematopoietic Neoplasms3. In the process of revising the morphology section, a Field Trial version was published and tested in both the United States and Europe. Epidemiologists, statisticians, and oncologists, as well as cancer registrars, are interested in studying trends in both incidence and mortality.