Comparative Anatomy and Evolution of the Odontocete Forelimb
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Record of Carcharocles Megalodon in the Eastern
Estudios Geológicos julio-diciembre 2015, 71(2), e032 ISSN-L: 0367-0449 doi: http://dx.doi.org/10.3989/egeol.41828.342 Record of Carcharocles megalodon in the Eastern Guadalquivir Basin (Upper Miocene, South Spain) Registro de Carcharocles megalodon en el sector oriental de la Cuenca del Guadalquivir (Mioceno superior, Sur de España) M. Reolid, J.M. Molina Departamento de Geología, Universidad de Jaén, Campus Las Lagunillas sn, 23071 Jaén, Spain. Email: [email protected] / [email protected] ABSTRACT Tortonian diatomites of the San Felix Quarry (Porcuna), in the Eastern Guadalquivir Basin, have given isolated marine vertebrate remains that include a large shark tooth (123.96 mm from apex to the baseline of the root). The large size of the crown height (92.2 mm), the triangular shape, the broad serrated crown, the convex lingual face and flat labial face, and the robust, thick angled root determine that this specimen corresponds to Carcharocles megalodon. The symmetry with low slant shows it to be an upper anterior tooth. The total length estimated from the tooth crown height is calculated by means of different methods, and comparison is made with Carcharodon carcharias. The final inferred total length of around 11 m classifies this specimen in the upper size range of the known C. megalodon specimens. The palaeogeography of the Guadalquivir Basin close to the North Betic Strait, which connected the Atlantic Ocean to the Mediterranean Sea, favoured the interaction of the cold nutrient-rich Atlantic waters with warmer Mediterranean waters. The presence of diatomites indicates potential upwelling currents in this context, as well as high productivity favouring the presence of large vertebrates such as mysticetid whales, pinnipeds and small sharks (Isurus). -

Cetacea, Odontoceti, Physeteridae) Del Mioceno Superior, Sector Occidental De La Cuenca Del Guadalquivir (Sur De España)
436 RevistaToscano Mexicana et al. de Ciencias Geológicas, v. 30, núm. 2, 2013, p. 436-445 Nuevos restos de Scaldicetus (Cetacea, Odontoceti, Physeteridae) del Mioceno superior, sector occidental de la Cuenca del Guadalquivir (sur de España) Antonio Toscano1,*, Manuel Abad1, Francisco Ruiz1, Fernando Muñiz1, Genaro Álvarez2, Edith Xio-Mara García3 y José Antonio Caro2 1 Departamento Geodinámica y Paleontología, Facultad de Ciencias Experimentales, Universidad de Huelva, Av. 3 de Marzo s/n, 21071 Huelva, España. 2 Sociedad Espeleológica Geos (Exploraciones e Investigaciones Subterráneas), 41080 Sevilla, España. 3 Centro Universitario de Ciencias Biológicas y Agropecuarias, Universidad de Guadalajara, Km. 15.5 Carretera a Nogales, Predio Las Agujas, Zapopan, Jalisco, México. *[email protected] RESUMEN La familia Physeteridae constituye una rama basal dentro de los cetáceos odontocetos. En la actualidad solo está representada por tres especies pertenecientes a los géneros Physeter y Kogia, aunque incluye numerosos géneros extintos. Scaldicetus es uno de los géneros neógenos más comunes y problemáticos desde el punto de vista taxonómico de la extinta subfamilia Hoplocetinae. Considerado un género polifilético, su uso sistemático ha quedado relegado a piezas dentales aisladas. En este trabajo se analizan dos piezas dentales atribuidas al género Scaldicetus halladas en dos formaciones del Mioceno superior de la región occidental de la Cuenca del Guadalquivir (sur de España). Los análisis sedimentológico y paleontológico de las facies indican ambientes litorales y marinos profundos, con abundantes presas potenciales para Scaldicetus. Sus características morfológicas y su distribución ecológica y paleobiogeográfica, indican que Scaldicetus ocupó un nicho ecológico similar al de Orcinus orca en la actualidad. Esta aportación constituye la primera revisión sistemática de restos fósiles de odontocetos en el sur de España, e intenta aportar información que contribuya a la comprensión de este controvertido género. -

First Description of a Tooth of the Extinct Giant Shark Carcharocles
First description of a tooth of the extinct giant shark Carcharocles megalodon (Agassiz, 1835) found in the province of Seville (SW Iberian Peninsula) (Otodontidae) Primera descripción de un diente del extinto tiburón gigante Carcharocles megalodon (Agassiz, 1835) encontrado en la provincia de Sevilla (SO de la Península Ibérica) (Otodontidae) José Luis Medina-Gavilán 1, Antonio Toscano 2, Fernando Muñiz 3, Francisco Javier Delgado 4 1. Sociedad de Estudios Ambientales (SOCEAMB) − Perú 4, 41100 Coria del Río, Sevilla (Spain) − [email protected] 2. Departamento de Geodinámica y Paleontología, Facultad de Ciencias Experimentales, Universidad de Huelva − Campus El Carmen, 21071 Huelva (Spain) − [email protected] 3. Departamento de Geodinámica y Paleontología, Facultad de Ciencias Experimentales, Universidad de Huelva − Campus El Carmen, 21071 Huelva (Spain) − [email protected] 4. Usuario de BiodiversidadVirtual.org − Álvarez Quintero 13, 41220 Burguillos, Sevilla (Spain) − [email protected] ABSTRACT: Fossil remains of the extinct giant shark Carcharocles megalodon (Agassiz, 1835) are rare in interior Andalusia (Southern Spain). For the first time, a fossil tooth belonging to this paleospecies is described from material found in the province of Seville (Burguillos). KEY WORDS: Carcharocles megalodon (Agassiz, 1835), megalodon, Otodontidae, fossil, paleontology, Burguillos, Seville, Tortonian. RESUMEN: Los restos del extinto tiburón gigante Carcharocles megalodon (Agassiz, 1835) son raros en el interior de Andalucía (sur de España). Por primera vez, se describe un diente fósil de esta paleoespecie a partir de material hallado en Sevilla (Burguillos). PALABRAS CLAVE: Carcharocles megalodon (Agassiz, 1835), megalodón, Otodontidae, fósil, paleontología, Burguillos, Sevilla, Tortoniense. Introduction Carcharocles megalodon (Agassiz, 1835), the megalodon, is widely recognised as the largest shark that ever lived. -

New Finds of Giant Raptorial Sperm Whale Teeth (Cetacea, Physeteroidea) from the Westerschelde Estuary (Province of Zeeland, the Netherlands)
1 Online Journal of the Natural History Museum Rotterdam, with contributions on zoology, paleontology and urban ecology deinsea.nl New finds of giant raptorial sperm whale teeth (Cetacea, Physeteroidea) from the Westerschelde Estuary (province of Zeeland, the Netherlands) Jelle W.F. Reumer 1,2, Titus H. Mens 1 & Klaas Post 2 1 Utrecht University, Faculty of Geosciences, P.O. Box 80115, 3508 TC Utrecht, the Netherlands 2 Natural History Museum Rotterdam, Westzeedijk 345 (Museumpark), 3015 AA Rotterdam, the Netherlands ABSTRACT Submitted 26 June 2017 Two large sperm whale teeth were found offshore from Breskens in the Westerschelde Accepted 28 July 2017 estuary. Comparison shows they share features with the teeth of the stem physteroid Published 23 August 2017 Zygophyseter, described from the Late Miocene of southern Italy. Both teeth are however significantly larger than the teeth of theZygophyseter type material, yet still somewhat Author for correspondence smaller than the teeth of the giant raptorial sperm whale Livyatan melvillei, and confirm the Jelle W.F. Reumer: presence of so far undescribed giant macroraptorial sperm whales in the Late Miocene of [email protected] The Netherlands. Editors of this paper Keywords Cetacea, Odontoceti, Westerschelde, Zygophyseter Bram W. Langeveld C.W. (Kees) Moeliker Cite this article Reumer, J.W.F., Mens, T.H. & Post, K. 2017 - New finds of giant raptorial sperm whale teeth (Cetacea, Physeteroidea) from the Westerschelde Estuary (province of Copyright Zeeland, the Netherlands) - Deinsea 17: 32 - 38 2017 Reumer, Mens & Post Distributed under Creative Commons CC-BY 4.0 DEINSEA online ISSN 2468-8983 INTRODUCTION presence of teeth in both maxilla and mandibula they are iden- Fossil Physeteroidea are not uncommon in Neogene marine tified as physeteroid teeth (Gol’din & Marareskul 2013). -

How Plesiosaurs Swam: New Insights Into Their Underwater Flight Using “Ava”, a Virtual Pliosaur
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 9 October 2019 doi:10.20944/preprints201910.0094.v1 How Plesiosaurs Swam: New Insights into Their Underwater Flight Using “Ava”, a Virtual Pliosaur Max Hawthorne1,*, Mark A. S. McMenamin 2, Paul de la Salle3 1Far From The Tree Press, LLC, 4657 York Rd., #952, Buckingham, PA, 18912, United States 2Department of Geology and Geography, Mount Holyoke College, South Hadley, Massachusetts, United States 3Swindon, England *Correspondence: [email protected]; Tel.: 267-337-7545 Abstract Analysis of plesiosaur swim dynamics by means Further study attempted to justify the use of all four flippers of a digital 3D armature (wireframe “skeleton”) of a simultaneously via the use of paddle-generated vortices, pliosauromorph (“Ava”) demonstrates that: 1, plesiosaurs which require specific timing to achieve optimal additional used all four flippers for primary propulsion; 2, plesiosaurs thrust. These attempts have largely relied on anatomical utilized all four flippers simultaneously; 3, respective pairs studies of strata-compressed plesiosaur skeletons, and/or of flippers of Plesiosauridae, front and rear, traveled through preconceived notions as pertains to the paddles’ inherent distinctive, separate planes of motion, and; 4, the ability to ranges of motion [8, 10-12]. What has not been considered utilize all four paddles simultaneously allowed these largely are the opposing angles of the pectoral and pelvic girdles, predatory marine reptiles to achieve a significant increase in which strongly indicate varied-yet-complementing relations acceleration and speed, which, in turn, contributed to their between the front and rear sets of paddles, both in repose and sustained dominance during the Mesozoic. -
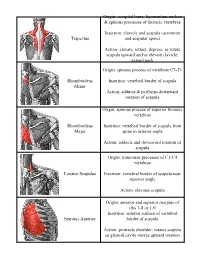
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Systematic Morphology of Fishes in the Early 21St Century
Copeia 103, No. 4, 2015, 858–873 When Tradition Meets Technology: Systematic Morphology of Fishes in the Early 21st Century Eric J. Hilton1, Nalani K. Schnell2, and Peter Konstantinidis1 Many of the primary groups of fishes currently recognized have been established through an iterative process of anatomical study and comparison of fishes that has spanned a time period approaching 500 years. In this paper we give a brief history of the systematic morphology of fishes, focusing on some of the individuals and their works from which we derive our own inspiration. We further discuss what is possible at this point in history in the anatomical study of fishes and speculate on the future of morphology used in the systematics of fishes. Beyond the collection of facts about the anatomy of fishes, morphology remains extremely relevant in the age of molecular data for at least three broad reasons: 1) new techniques for the preparation of specimens allow new data sources to be broadly compared; 2) past morphological analyses, as well as new ideas about interrelationships of fishes (based on both morphological and molecular data) provide rich sources of hypotheses to test with new morphological investigations; and 3) the use of morphological data is not limited to understanding phylogeny and evolution of fishes, but rather is of broad utility to understanding the general biology (including phenotypic adaptation, evolution, ecology, and conservation biology) of fishes. Although in some ways morphology struggles to compete with the lure of molecular data for systematic research, we see the anatomical study of fishes entering into a new and exciting phase of its history because of recent technological and methodological innovations. -

Comparative Anatomy and Functional Morphology University of California at Berkeley Department of Integrative Biology (5 Units)
Comparative Anatomy and Functional Morphology University of California at Berkeley Department of Integrative Biology (5 Units) Course Description: This course is an in-depth look at the biology of form and function. We will examine vertebrate anatomy to understand how structures develop, how they have evolved, and how they interact with one another to allow animals to live in a variety of environments. We will study the integration of the skeletal, muscular, nervous, vascular, respiratory, digestive, endocrine, and urogenital systems to explore the historical and present diversity of vertebrate animals. Format: This course will include lecture, discussion, and laboratory sessions. Lectures will focus primarily on broad concepts and ideas. Discussion sections will emphasize how to both read and analyze primary scientific literature. Laboratory sessions will allow students to physically explore the morphologies of a diversity of taxa and will include museum specimens as well as dissections of whole animals. Lecture: Monday, Tuesday, Wednesday, and Thursday, 9:30-11:00a.m. Discussion: Monday and Thursday, 11:00a.m. or 12:00p.m. Laboratory: Monday, Tuesday, Wednesday, and Thursday, 2:00-5:00p.m. All students must take lectures, discussion sections, and laboratory sections concurrently. Attendance of all sections is required. Goals: 1. A comparative approach will allow students to gain experience in identifying similarities and differences among taxa and further their understanding of how both evolutionary and environmental contexts influence the morphology and function. 2. Students will improve their ability to ask questions and answer them using scientific methodology. 3. Students will gain factual knowledge of terms and concepts regarding vertebrate anatomy and functional morphology. -

Coracoid Process Anatomy: a Cadaveric Study of Surgically Relevant Structures Jorge Chahla, M.D., Ph.D., Daniel Cole Marchetti, B.A., Gilbert Moatshe, M.D., Márcio B
Quantitative Assessment of the Coracoacromial and the Coracoclavicular Ligaments With 3-Dimensional Mapping of the Coracoid Process Anatomy: A Cadaveric Study of Surgically Relevant Structures Jorge Chahla, M.D., Ph.D., Daniel Cole Marchetti, B.A., Gilbert Moatshe, M.D., Márcio B. Ferrari, M.D., George Sanchez, B.S., Alex W. Brady, M.Sc., Jonas Pogorzelski, M.D., M.H.B.A., George F. Lebus, M.D., Peter J. Millett, M.D., M.Sc., Robert F. LaPrade, M.D., Ph.D., and CAPT Matthew T. Provencher, M.D., M.C., U.S.N.R. Purpose: To perform a quantitative anatomic evaluation of the (1) coracoid process, specifically the attachment sites of the conjoint tendon, the pectoralis minor, the coracoacromial ligament (CAL), and the coracoclavicular (CC) ligaments in relation to pertinent osseous and soft tissue landmarks; (2) CC ligaments’ attachments on the clavicle; and (3) CAL attachment on the acromion in relation to surgically relevant anatomic landmarks to assist in planning of the Latarjet procedure, acromioclavicular (AC) joint reconstructions, and CAL resection distances avoiding iatrogenic injury to sur- rounding structures. Methods: Ten nonpaired fresh-frozen human cadaveric shoulders (mean age 52 years, range 33- 64 years) were included in this study. A 3-dimensional coordinate measuring device was used to quantify the location of pertinent bony landmarks and soft tissue attachment areas. The ligament and tendon attachment perimeters and center points on the coracoid, clavicle, and acromion were identified and subsequently dissected off the bone. Coordinates of points along the perimeters of attachment sites were used to calculate areas, whereas coordinates of center points were used to determine distances between surgically relevant attachment sites and pertinent bony landmarks. -

Dolphin P-K Teacher's Guide
Dolphin P-K Teacher’s Guide Table of Contents ii Goal and Objectives iii Message to Our Teacher Partners 1 Dolphin Overview 3 Dolphin Activities 23 Dolphin Discovery Dramatic Play 7 Which Animals Live with 25 Dolphins? Picture This: Dolphin Mosaic 9 Pod Count 27 Dolphins on the Move 13 How Do They Measure Up? 31 Where Do I Live? Food Search 15 dorsal dorsal 35 Dolphin or fin peduncle Other Sea Creature? median blowhole notch posterior Build a anterior fluke17s melon 37 pectoral Dolphin Recycling flipper eye rostrum ear Can Make a bottlenose dolphin Difference! 19 ventral lengDolphinth = 10-14 feet / 3-4.2 meters Hokeypokey 41 d Vocabularyi p h o l n Goal and Message to Our Objectives Teacher Partners At l a n t i s , Paradise Island, strives to inspire students to learn Goal: Students will develop an understanding of what a more about the ocean that surrounds dolphin is and where it lives. them in The Bahamas. Through interactive, interdisciplinary activities in the classroom and at Atlantis, we endeavor to help students develop an understanding of the marine world along with Upon the completion of the Dolphin W e a r e the desire to conserve it and its wildlife. Dolphin Cay Objectives: provides students with a thrilling and inspirational program, students will be able to: a resource for you. Atlantis, Paradise Island, offers opportunity to learn about dolphins and their undersea a variety of education programs on world as well as ways they can help conserve them. themes such as dolphins, coral reefs, sharks, Through students’ visit to Atlantis, we hope to Determine which animals live in the ocean like dolphins. -

The Devonian Tetrapod Acanthostega Gunnari Jarvik: Postcranial Anatomy, Basal Tetrapod Interrelationships and Patterns of Skeletal Evolution M
Transactions of the Royal Society of Edinburgh: Earth Sciences, 87, 363-421, 1996 The Devonian tetrapod Acanthostega gunnari Jarvik: postcranial anatomy, basal tetrapod interrelationships and patterns of skeletal evolution M. I. Coates ABSTRACT: The postcranial skeleton of Acanthostega gunnari from the Famennian of East Greenland displays a unique, transitional, mixture of features conventionally associated with fish- and tetrapod-like morphologies. The rhachitomous vertebral column has a primitive, barely differentiated atlas-axis complex, encloses an unconstricted notochordal canal, and the weakly ossified neural arches have poorly developed zygapophyses. More derived axial skeletal features include caudal vertebral proliferation and, transiently, neural radials supporting unbranched and unsegmented lepidotrichia. Sacral and post-sacral ribs reiterate uncinate cervical and anterior thoracic rib morphologies: a simple distal flange supplies a broad surface for iliac attachment. The octodactylous forelimb and hindlimb each articulate with an unsutured, foraminate endoskeletal girdle. A broad-bladed femoral shaft with extreme anterior torsion and associated flattened epipodials indicates a paddle-like hindlimb function. Phylogenetic analysis places Acanthostega as the sister- group of Ichthyostega plus all more advanced tetrapods. Tulerpeton appears to be a basal stem- amniote plesion, tying the amphibian-amniote split to the uppermost Devonian. Caerorhachis may represent a more derived stem-amniote plesion. Postcranial evolutionary trends spanning the taxa traditionally associated with the fish-tetrapod transition are discussed in detail. Comparison between axial skeletons of primitive tetrapods suggests that plesiomorphic fish-like morphologies were re-patterned in a cranio-caudal direction with the emergence of tetrapod vertebral regionalisation. The evolution of digited limbs lags behind the initial enlargement of endoskeletal girdles, whereas digit evolution precedes the elaboration of complex carpal and tarsal articulations. -

Reconstructing Pectoral Appendicular Muscle Anatomy in Fossil Fish and Tetrapods Over the Fins-To-Limbs Transition
Biol. Rev. (2017), pp. 000–000. 1 doi: 10.1111/brv.12386 Reconstructing pectoral appendicular muscle anatomy in fossil fish and tetrapods over the fins-to-limbs transition Julia L. Molnar1,∗ , Rui Diogo2, John R. Hutchinson3 and Stephanie E. Pierce4 1Department of Anatomy, New York Institute of Technology College of Osteopathic Medicine, Northern Boulevard, Old Westbury, NY, U.S.A. 2Department of Anatomy, Howard University College of Medicine, 520 W St. NW, Numa Adams Building, Washington, DC 20059, U.S.A. 3Structure and Motion Lab, Royal Veterinary College, Hawkshead Lane, Hatfield, Hertfordshire AL9 7TA, UK 4Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, U.S.A. ABSTRACT The question of how tetrapod limbs evolved from fins is one of the great puzzles of evolutionary biology. While palaeontologists, developmental biologists, and geneticists have made great strides in explaining the origin and early evolution of limb skeletal structures, that of the muscles remains largely unknown. The main reason is the lack of consensus about appendicular muscle homology between the closest living relatives of early tetrapods: lobe-finned fish and crown tetrapods. In the light of a recent study of these homologies, we re-examined osteological correlates of muscle attachment in the pectoral girdle, humerus, radius, and ulna of early tetrapods and their close relatives. Twenty-nine extinct and six extant sarcopterygians were included in a meta-analysis using information from the literature and from original specimens, when possible. We analysed these osteological correlates using parsimony-based character optimization in order to reconstruct muscle anatomy in ancestral lobe-finned fish, tetrapodomorph fish, stem tetrapods, and crown tetrapods.