Reconstructing Pectoral Appendicular Muscle Anatomy in Fossil Fish and Tetrapods Over the Fins-To-Limbs Transition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Tetrapod Relationships Revisited
Biol. Rev. (2003), 78, pp. 251–345. f Cambridge Philosophical Society 251 DOI: 10.1017/S1464793102006103 Printed in the United Kingdom Early tetrapod relationships revisited MARCELLO RUTA1*, MICHAEL I. COATES1 and DONALD L. J. QUICKE2 1 The Department of Organismal Biology and Anatomy, The University of Chicago, 1027 East 57th Street, Chicago, IL 60637-1508, USA ([email protected]; [email protected]) 2 Department of Biology, Imperial College at Silwood Park, Ascot, Berkshire SL57PY, UK and Department of Entomology, The Natural History Museum, Cromwell Road, London SW75BD, UK ([email protected]) (Received 29 November 2001; revised 28 August 2002; accepted 2 September 2002) ABSTRACT In an attempt to investigate differences between the most widely discussed hypotheses of early tetrapod relation- ships, we assembled a new data matrix including 90 taxa coded for 319 cranial and postcranial characters. We have incorporated, where possible, original observations of numerous taxa spread throughout the major tetrapod clades. A stem-based (total-group) definition of Tetrapoda is preferred over apomorphy- and node-based (crown-group) definitions. This definition is operational, since it is based on a formal character analysis. A PAUP* search using a recently implemented version of the parsimony ratchet method yields 64 shortest trees. Differ- ences between these trees concern: (1) the internal relationships of aı¨stopods, the three selected species of which form a trichotomy; (2) the internal relationships of embolomeres, with Archeria -
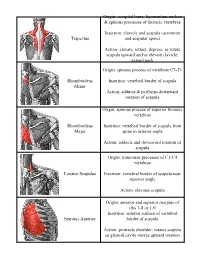
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Coracoid Process Anatomy: a Cadaveric Study of Surgically Relevant Structures Jorge Chahla, M.D., Ph.D., Daniel Cole Marchetti, B.A., Gilbert Moatshe, M.D., Márcio B
Quantitative Assessment of the Coracoacromial and the Coracoclavicular Ligaments With 3-Dimensional Mapping of the Coracoid Process Anatomy: A Cadaveric Study of Surgically Relevant Structures Jorge Chahla, M.D., Ph.D., Daniel Cole Marchetti, B.A., Gilbert Moatshe, M.D., Márcio B. Ferrari, M.D., George Sanchez, B.S., Alex W. Brady, M.Sc., Jonas Pogorzelski, M.D., M.H.B.A., George F. Lebus, M.D., Peter J. Millett, M.D., M.Sc., Robert F. LaPrade, M.D., Ph.D., and CAPT Matthew T. Provencher, M.D., M.C., U.S.N.R. Purpose: To perform a quantitative anatomic evaluation of the (1) coracoid process, specifically the attachment sites of the conjoint tendon, the pectoralis minor, the coracoacromial ligament (CAL), and the coracoclavicular (CC) ligaments in relation to pertinent osseous and soft tissue landmarks; (2) CC ligaments’ attachments on the clavicle; and (3) CAL attachment on the acromion in relation to surgically relevant anatomic landmarks to assist in planning of the Latarjet procedure, acromioclavicular (AC) joint reconstructions, and CAL resection distances avoiding iatrogenic injury to sur- rounding structures. Methods: Ten nonpaired fresh-frozen human cadaveric shoulders (mean age 52 years, range 33- 64 years) were included in this study. A 3-dimensional coordinate measuring device was used to quantify the location of pertinent bony landmarks and soft tissue attachment areas. The ligament and tendon attachment perimeters and center points on the coracoid, clavicle, and acromion were identified and subsequently dissected off the bone. Coordinates of points along the perimeters of attachment sites were used to calculate areas, whereas coordinates of center points were used to determine distances between surgically relevant attachment sites and pertinent bony landmarks. -

The Devonian Tetrapod Acanthostega Gunnari Jarvik: Postcranial Anatomy, Basal Tetrapod Interrelationships and Patterns of Skeletal Evolution M
Transactions of the Royal Society of Edinburgh: Earth Sciences, 87, 363-421, 1996 The Devonian tetrapod Acanthostega gunnari Jarvik: postcranial anatomy, basal tetrapod interrelationships and patterns of skeletal evolution M. I. Coates ABSTRACT: The postcranial skeleton of Acanthostega gunnari from the Famennian of East Greenland displays a unique, transitional, mixture of features conventionally associated with fish- and tetrapod-like morphologies. The rhachitomous vertebral column has a primitive, barely differentiated atlas-axis complex, encloses an unconstricted notochordal canal, and the weakly ossified neural arches have poorly developed zygapophyses. More derived axial skeletal features include caudal vertebral proliferation and, transiently, neural radials supporting unbranched and unsegmented lepidotrichia. Sacral and post-sacral ribs reiterate uncinate cervical and anterior thoracic rib morphologies: a simple distal flange supplies a broad surface for iliac attachment. The octodactylous forelimb and hindlimb each articulate with an unsutured, foraminate endoskeletal girdle. A broad-bladed femoral shaft with extreme anterior torsion and associated flattened epipodials indicates a paddle-like hindlimb function. Phylogenetic analysis places Acanthostega as the sister- group of Ichthyostega plus all more advanced tetrapods. Tulerpeton appears to be a basal stem- amniote plesion, tying the amphibian-amniote split to the uppermost Devonian. Caerorhachis may represent a more derived stem-amniote plesion. Postcranial evolutionary trends spanning the taxa traditionally associated with the fish-tetrapod transition are discussed in detail. Comparison between axial skeletons of primitive tetrapods suggests that plesiomorphic fish-like morphologies were re-patterned in a cranio-caudal direction with the emergence of tetrapod vertebral regionalisation. The evolution of digited limbs lags behind the initial enlargement of endoskeletal girdles, whereas digit evolution precedes the elaboration of complex carpal and tarsal articulations. -

Breathing and Locomotion in Birds
Breathing and locomotion in birds. A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Life Sciences. 2010 Peter George Tickle Contents Abstract 4 Declaration 5 Copyright Statement 6 Author Information 7 Acknowledgements 9 Organisation of this PhD thesis 10 Chapter 1 General Introduction 13 1. Introduction 14 1.1 The Avian Respiratory System 14 1.1.1 Structure of the lung and air sacs 16 1.1.2 Airflow in the avian respiratory system 21 1.1.3 The avian aspiration pump 25 1.2 The uncinate processes in birds 29 1.2.1 Uncinate process morphology and biomechanics 32 1.3 Constraints on breathing in birds 33 1.3.1 Development 33 1.3.2 Locomotion 35 1.3.2.1 The appendicular skeleton 35 1.3.2.2 Overcoming the trade-off between breathing 36 and locomotion 1.3.2.3 Energetics of locomotion in birds 38 1.4 Evolution of the ventilatory pump in birds 41 1.5 Overview and Thesis Aims 42 2 Chapter 2 Functional significance of the uncinate processes in birds. 44 Chapter 3 Ontogenetic development of the uncinate processes in the 45 domestic turkey (Meleagris gallopavo). Chapter 4 Uncinate process length in birds scales with resting metabolic rate. 46 Chapter 5 Load carrying during locomotion in the barnacle goose (Branta 47 leucopsis): The effect of load placement and size. Chapter 6 A continuum in ventilatory mechanics from early theropods to 48 extant birds. Chapter 7 General Discussion 49 References 64 3 Abstract of a thesis by Peter George Tickle submitted to the University of Manchester for the degree of PhD in the Faculty of Life Sciences and entitled ‘Breathing and Locomotion in Birds’. -

Annual Meeting 2002
Newsletter 51 74 Newsletter 51 75 The Palaeontological Association 46th Annual Meeting 15th–18th December 2002 University of Cambridge ABSTRACTS Newsletter 51 76 ANNUAL MEETING ANNUAL MEETING Newsletter 51 77 Holocene reef structure and growth at Mavra Litharia, southern coast of Gulf of Corinth, Oral presentations Greece: a simple reef with a complex message Steve Kershaw and Li Guo Oral presentations will take place in the Physiology Lecture Theatre and, for the parallel sessions at 11:00–1:00, in the Tilley Lecture Theatre. Each presentation will run for a New perspectives in palaeoscolecidans maximum of 15 minutes, including questions. Those presentations marked with an asterisk Oliver Lehnert and Petr Kraft (*) are being considered for the President’s Award (best oral presentation by a member of the MONDAY 11:00—Non-marine Palaeontology A (parallel) Palaeontological Association under the age of thirty). Guts and Gizzard Stones, Unusual Preservation in Scottish Middle Devonian Fishes Timetable for oral presentations R.G. Davidson and N.H. Trewin *The use of ichnofossils as a tool for high-resolution palaeoenvironmental analysis in a MONDAY 9:00 lower Old Red Sandstone sequence (late Silurian Ringerike Group, Oslo Region, Norway) Neil Davies Affinity of the earliest bilaterian embryos The harvestman fossil record Xiping Dong and Philip Donoghue Jason A. Dunlop Calamari catastrophe A New Trigonotarbid Arachnid from the Early Devonian Windyfield Chert, Rhynie, Philip Wilby, John Hudson, Roy Clements and Neville Hollingworth Aberdeenshire, Scotland Tantalizing fragments of the earliest land plants Steve R. Fayers and Nigel H. Trewin Charles H. Wellman *Molecular preservation of upper Miocene fossil leaves from the Ardeche, France: Use of Morphometrics to Identify Character States implications for kerogen formation Norman MacLeod S. -

I Ecomorphological Change in Lobe-Finned Fishes (Sarcopterygii
Ecomorphological change in lobe-finned fishes (Sarcopterygii): disparity and rates by Bryan H. Juarez A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science (Ecology and Evolutionary Biology) in the University of Michigan 2015 Master’s Thesis Committee: Assistant Professor Lauren C. Sallan, University of Pennsylvania, Co-Chair Assistant Professor Daniel L. Rabosky, Co-Chair Associate Research Scientist Miriam L. Zelditch i © Bryan H. Juarez 2015 ii ACKNOWLEDGEMENTS I would like to thank the Rabosky Lab, David W. Bapst, Graeme T. Lloyd and Zerina Johanson for helpful discussions on methodology, Lauren C. Sallan, Miriam L. Zelditch and Daniel L. Rabosky for their dedicated guidance on this study and the London Natural History Museum for courteously providing me with access to specimens. iii TABLE OF CONTENTS ACKNOWLEDGEMENTS ii LIST OF FIGURES iv LIST OF APPENDICES v ABSTRACT vi SECTION I. Introduction 1 II. Methods 4 III. Results 9 IV. Discussion 16 V. Conclusion 20 VI. Future Directions 21 APPENDICES 23 REFERENCES 62 iv LIST OF TABLES AND FIGURES TABLE/FIGURE II. Cranial PC-reduced data 6 II. Post-cranial PC-reduced data 6 III. PC1 and PC2 Cranial and Post-cranial Morphospaces 11-12 III. Cranial Disparity Through Time 13 III. Post-cranial Disparity Through Time 14 III. Cranial/Post-cranial Disparity Through Time 15 v LIST OF APPENDICES APPENDIX A. Aquatic and Semi-aquatic Lobe-fins 24 B. Species Used In Analysis 34 C. Cranial and Post-Cranial Landmarks 37 D. PC3 and PC4 Cranial and Post-cranial Morphospaces 38 E. PC1 PC2 Cranial Morphospaces 39 1-2. -

Some Notes on the Diverse Brachiosaurid Sauropods of the Late Jurassic of North America, Europe and Africa
Some Notes on the Diverse Brachiosaurid Sauropods of the Late Jurassic of North America, Europe and Africa GREGORY S. PAUL 3109 N. Calvert St. Baltimore, Maryland 21218 USA [email protected] June 2012 Abstract: The unusual placement of the parapophyses on elongotated peduncles further distinguishes Brachiosaurus from other brachiosaurid genera including Giraffatitan. Late Jurassic brachiosaurid taxonomy is probably more complex than has been realized, in part because of evolution over the considerable periods of times recorded in the Morrison and Tendaguru formations. The presence of reduced tails on late Jurassic brachiosaurids is confirmed. INTRODUCTION It was long assumed that the brachiosaurid material found in the Late Jurassic Morrison, Tendaguru, and Lourinha Formations from three continents belonged to the genus Brachiosaurus (as per Janensch 1950, 1961, Lapparent and Zbyszewski 1957, Jensen 1987) until Paul (1988) noted significant differences between the type species B. altithorax (Fig. 1A) from North America and the African B. brancai (Fig. 1B) indicated at least a subgeneric separation, and Taylor (2009) formally separated the sauropods, leaving much of the Tendaguru brachiosaurids material titled Giraffatitan brancai; the generic separation has been tentatively questioned (Chure et al. 2010). In addition European B. alatalaiensis has been retitled Lusotitan alatalaiensis (Antunes and Mateus 2003), and a dwarf brachiosaur from the European Mittlere Kimmeridge-Stufe deposits has been designated Europasaurus holgeri (Sander et al. 2006). Institutional Abbreviations: AMNH, American Museum of Natural History, New York; BYU, Brigham Young University, Provo; FMNH, Field Museum of Natural History, Chicago; HMN, Humboldt Museum fur Naturkunde, Berlin; NHMUK, Natural History Museum, London; USNM, United States Natural History Museum, Washington DC. -

Rehabilitation Guidelines for Latarjet/Coracoid Process Transfer Josef K
Rehabilitation Guidelines for Latarjet/Coracoid Process Transfer Josef K. Eichinger, MD Shoulder instability may be caused from congenital deformity, recurrent overuse activity, and/or traumatic dislocation. Surgical stabilization of the glenohumeral joint is indicated after conservative treatment fails and recurrent instability/subluxation continues. A number of different surgical procedures may be indicated in this situation, often divided into soft tissue or bony procedures. Shoulder Instability – Soft Tissue: Surgical reconstruction targeting the glenohumeral joint’s soft tissues for shoulder instability, typically involves labral repairs, the most common being the Bankart repair. A Bankart lesion typically occurs from an anterior-inferior dislocation of the humerus, tearing the labrum from it’s attachment to the glenoid, thereby detaching the inferior gleno-humeral ligament (IGHL). Surgical management of this revolves around labral repair to reattach the IGHL under appropriate tension. This may be accomplished either arthroscopically or through an open approach.1 Most traumatic glenohumeral dislocations may not only cause a Bankart lesion, but may create impression fractures in the postero-superior humeral head termed Hill-Sachs lesions.2 An adverse effect from this procedure includes suturing the capsule too tightly, causing a shortening of the capsule, and thus decreasing the external rotation allowed at the glenohumeral joint. Other complications are extremely rare, but may include axillary nerve damage, subscapularis rupture (seen only in open repairs performed with subscapularis detachment and repair), and recurrent instability. If there is bony deficiency in the glenoid, which represents 20% or more of the antero-inferior glenoid, it is biomechanically impossible to restore the same stability and is therefore more prone to recurrent instability and failure. -

Coracoid Fractures and Dislocations
Coracoid Fractures and Dislocations Brett Gartrell Wildbase, Institute of Veterinary, Animal and Biomedical Sciences Massey University, Palmerston North, New Zealand ABSTRACT The treatment of coracoid dislocations and fractures in the New Zealand native wood pigeon (kereru; Hemiphaga novaeseelandiae), undertaken at the Wildbase Hospital at Massey University from 2002‐ 2012. After briefly reviewing the functional anatomy of the coracoid bones, the results of research on the nature of collisions that cause such injuries are reviewed. A comparative assessment of the relative force of collisions dependent on the body size of the bird is presented. The complications of coracoid fractures and dislocations including pulmonary haemorrhage, aspergillosis, cardiac contusions and rhythm disturbances is discussed. A new technique for the repair of coracoid dislocations from the keel and intramedullary pinning for repair of coracoid fractures are outlined. The merits of surgical versus conservative treatment of these injuries are discussed. ANATOMY The coracoid bones are essential to flight in birds and a reduced version of them is present in avian ancestors including Archaeopteryx, the earliest known flying bird from the Late Jurassic period (Figure 1) (Baier et al., 2007). Figure 1. Diagrammatic reconstruction of Archaeopteryx skeleton showing the modified coracoid bone (arrow) attached to the limited keel. Image modified from (Rowe, 2011). The function of the avian shoulder in flight is a complex interplay of forces from muscles, tendons and ligaments acting on the structural skeletal framework of coracoids, scapula, clavicles, humerus and keel. The coracoid bones are strut‐like support bones running from the shoulder to the keel, whose function is to resist the compressive forces of the pectoral muscle contraction (Figure 2). -

Tiktaalik—A Fishy 'Missing Link'
Countering the Critics Tiktaalik—a fishy ‘missing link’ Jonathan Sarfati he secularized mainstream media (MSM) are gleefully Is it transitional? promoting a recent find, Tiktaalik roseae (figure 1), as T Clack and others are naturally enthusiastic about Tikta- the end of any creationist or intelligent design idea. Some paleontologists are claiming that this is ‘a link between fishes alik’s transitional status. But this is not surprising—to her, and land vertebrates that might in time become as much of an we are all fishes anyway! She states: evolutionary icon as the proto-bird Archaeopteryx.’1 ‘Although humans do not usually think of So is Tiktaalik real evidence that fish evolved into tetra- themselves as fishes, they nonetheless share several pods (four-limbed vertebrates, i.e. amphibians, reptiles, mam- fundamental characters that unite them inextricably mals and birds)? As will be shown, there are parallels with with their relatives among the fishes … Tetrapods Archaeopteryx, the famous alleged reptile-bird intermediate, did not evolve from sarcopterygians [lobe-finned but not in the way the above quote claims! fishes]; theyare sarcopterygians, just as one would The alleged fish-to-tetrapod evolutionary transition is full of difficulties.2 In this, it parallels the record of dinosaur-to-bird,3 mammal-like reptiles,4 land-mam- mal-to-whale5 and ape-to-human evolution;6 superficially plausible, but when analyzed in depth, it col- lapses, for many parallel reasons. What was found? The above quote comes from two leading European experts in the alleged evolutionary transition from fish to tetrapod, Per Ahlberg and Jennifer Clack. -

Fins to Limbs: What the Fossils Say1
EVOLUTION & DEVELOPMENT 4:5, 390–401 (2002) Fins to limbs: what the fossils say1 Michael I. Coates,a,* Jonathan E. Jeffery,b and Marcello Rutaa aDepartment of Organismal Biology and Anatomy, University of Chicago, 1027 E57th Street, Chicago, IL 60637, USA bInstitute of Evolutionary and Ecological Sciences, Leiden University, Kaiserstraat 63, Postbus 9516, 2300 RA Leiden, The Netherlands *Author for correspondence (email: [email protected]) 1From the symposium on Starting from Fins: Parallelism in the Evolution of Limbs and Genitalia. SUMMARY A broad phylogenetic review of fins, limbs, and highlight a large data gap in the stem group preceding the first girdles throughout the stem and base of the crown group is appearance of limbs with digits. It is also noted that the record needed to get a comprehensive idea of transformations unique of morphological diversity among stem tetrapods is somewhat to the assembly of the tetrapod limb ground plan. In the lower worse than that of basal crown group tetrapods. The pre-limbed part of the tetrapod stem, character state changes at the pecto- evolution of stem tetrapod paired fins is marked by a gradual re- ral level dominate; comparable pelvic level data are limited. In duction in axial segment numbers (mesomeres); pectoral fins of more crownward taxa, pelvic level changes dominate and re- the sister group to limbed tetrapods include only three. This re- peatedly precede similar changes at pectoral level. Concerted duction in segment number is accompanied by increased re- change at both levels appears to be the exception rather than gional specialization, and these changes are discussed with the rule.