Breathing and Locomotion in Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3H?;Lnbcha Nb? 1?=L?Nm I@
3H?;LNBCHANB?1?=L?NMI@ #>O=;NIL%OC>? 1?=IH>#>CNCIH @C?F>GOM?OGILA13# 'HMC>? ;=EALIOH>'H@ILG;NCIH *?MMIHM@IL%L;>?M] >>CNCIH;F0?MIOL=?M 'HNLI>O=NCIH Unearthing the Secrets of SUE No dinosaur in the world compares to SUE—the largest, most complete, and best preserved Tyrannosaurus rex ever discovered. In May 2000, the unveiling of her 67-million-year-old skeleton at The Field Museum made global headlines. Since then, more than 16 million visitors have marveled over Chicago’s prehistoric giant. Using the story of SUE to captivate students’ imagination, the Unearthing the Secrets of SUE Educator Guide takes pre-k through eighth-grade students on an interactive exploration of SUE at The Field Museum and the scientific insights she’s providing about the world in which she lived. The lessons in this guide will engage students in the science of SUE by: • providing students unique access to SUE, the largest, most complete, and best preserved TYRANNOSAURUS REX ever discovered; • providing students with hands-on activities that enable them to investigate by making observations, developing hypotheses, questioning assumptions, testing ideas, and coming to conclusions; • introducing students to careers in science by highlighting the wide professional expertise involved in the SUE project; and • introducing students to the countless resources available to them through The Field Museum including field trips, and online research and interactive learning opportunities. How to Use this Guide • Detailed Background Information is provided to support educators in sharing the story of SUE with students. Use this information to prepare yourself and your students for learning about SUE. -

New Heterodontosaurid Remains from the Cañadón Asfalto Formation: Cursoriality and the Functional Importance of the Pes in Small Heterodontosaurids
Journal of Paleontology, 90(3), 2016, p. 555–577 Copyright © 2016, The Paleontological Society 0022-3360/16/0088-0906 doi: 10.1017/jpa.2016.24 New heterodontosaurid remains from the Cañadón Asfalto Formation: cursoriality and the functional importance of the pes in small heterodontosaurids Marcos G. Becerra,1 Diego Pol,1 Oliver W.M. Rauhut,2 and Ignacio A. Cerda3 1CONICET- Museo Palaeontológico Egidio Feruglio, Fontana 140, Trelew, Chubut 9100, Argentina 〈[email protected]〉; 〈[email protected]〉 2SNSB, Bayerische Staatssammlung für Paläontologie und Geologie and Department of Earth and Environmental Sciences, LMU München, Richard-Wagner-Str. 10, Munich 80333, Germany 〈[email protected]〉 3CONICET- Instituto de Investigación en Paleobiología y Geología, Universidad Nacional de Río Negro, Museo Carlos Ameghino, Belgrano 1700, Paraje Pichi Ruca (predio Marabunta), Cipolletti, Río Negro, Argentina 〈[email protected]〉 Abstract.—New ornithischian remains reported here (MPEF-PV 3826) include two complete metatarsi with associated phalanges and caudal vertebrae, from the late Toarcian levels of the Cañadón Asfalto Formation. We conclude that these fossil remains represent a bipedal heterodontosaurid but lack diagnostic characters to identify them at the species level, although they probably represent remains of Manidens condorensis, known from the same locality. Histological features suggest a subadult ontogenetic stage for the individual. A cluster analysis based on pedal measurements identifies similarities of this specimen with heterodontosaurid taxa and the inclusion of the new material in a phylogenetic analysis with expanded character sampling on pedal remains confirms the described specimen as a heterodontosaurid. Finally, uncommon features of the digits (length proportions among nonungual phalanges of digit III, and claw features) are also quantitatively compared to several ornithischians, theropods, and birds, suggesting that this may represent a bipedal cursorial heterodontosaurid with gracile and grasping feet and long digits. -

Ducks, Geese, and Swans of the World: Sources Cited
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Ducks, Geese, and Swans of the World by Paul A. Johnsgard Papers in the Biological Sciences 2010 Ducks, Geese, and Swans of the World: Sources Cited Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/biosciducksgeeseswans Part of the Ornithology Commons Johnsgard, Paul A., "Ducks, Geese, and Swans of the World: Sources Cited" (2010). Ducks, Geese, and Swans of the World by Paul A. Johnsgard. 17. https://digitalcommons.unl.edu/biosciducksgeeseswans/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Ducks, Geese, and Swans of the World by Paul A. Johnsgard by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Sources Cited Alder, L. P. 1963. The calls and displays of African and In Bellrose, F. C. 1976. Ducks, geese and swans of North dian pygmy geese. In Wildfowl Trust, 14th Annual America. 2d ed. Harrisburg, Pa.: Stackpole. Report, pp. 174-75. Bellrose, F. c., & Hawkins, A. S. 1947. Duck weights in Il Ali, S. 1960. The pink-headed duck Rhodonessa caryo linois. Auk 64:422-30. phyllacea (Latham). Wildfowl Trust, 11th Annual Re Bengtson, S. A. 1966a. [Observation on the sexual be port, pp. 55-60. havior of the common scoter, Melanitta nigra, on the Ali, S., & Ripley, D. 1968. Handbook of the birds of India breeding grounds, with special reference to courting and Pakistan, together with those of Nepal, Sikkim, parties.] Var Fagelvarld 25:202-26. -

Pterosaurs Flight in the Age of Dinosaurs Now Open 2 News at the Museum 3
Member Magazine Spring 2014 Vol. 39 No. 2 Pterosaurs Flight in the Age of Dinosaurs now open 2 News at the Museum 3 From the After an unseasonably cold, snowy winter, will work to identify items from your collection, More than 540,000 Marine Fossils the Museum is pleased to offer a number of while also displaying intriguing specimens from President springtime opportunities to awaken the inner the Museum’s own world-renowned collections. Added to Paleontology Collection naturalist in us all. This is the time of year when Of course, fieldwork and collecting have Ellen V. Futter Museum scientists prepare for the summer been hallmarks of the Museum’s work since Collections at a Glance field season as they continue to pursue new the institution’s founding. What has changed, discoveries in their fields. It’s also when Museum however, is technology. With a nod to the many Over nearly 150 years of acquisitions and Members and visitors can learn about their ways that technology is amplifying how scientific fieldwork, the Museum has amassed preeminent own discoveries during the annual Identification investigations are done, this year, ID Day visitors collections that form an irreplaceable record Day in Theodore Roosevelt Memorial Hall. can learn how scientists use digital fabrication of life on Earth. Today, 21st-century tools— Held this year on May 10, Identification Day to aid their research and have a chance to sophisticated imaging techniques, genomic invites visitors to bring their own backyard finds have their own objects scanned and printed on analyses, programs to analyze ever-growing and curios for identification by Museum scientists. -

Onetouch 4.0 Scanned Documents
/ Chapter 2 THE FOSSIL RECORD OF BIRDS Storrs L. Olson Department of Vertebrate Zoology National Museum of Natural History Smithsonian Institution Washington, DC. I. Introduction 80 II. Archaeopteryx 85 III. Early Cretaceous Birds 87 IV. Hesperornithiformes 89 V. Ichthyornithiformes 91 VI. Other Mesozojc Birds 92 VII. Paleognathous Birds 96 A. The Problem of the Origins of Paleognathous Birds 96 B. The Fossil Record of Paleognathous Birds 104 VIII. The "Basal" Land Bird Assemblage 107 A. Opisthocomidae 109 B. Musophagidae 109 C. Cuculidae HO D. Falconidae HI E. Sagittariidae 112 F. Accipitridae 112 G. Pandionidae 114 H. Galliformes 114 1. Family Incertae Sedis Turnicidae 119 J. Columbiformes 119 K. Psittaciforines 120 L. Family Incertae Sedis Zygodactylidae 121 IX. The "Higher" Land Bird Assemblage 122 A. Coliiformes 124 B. Coraciiformes (Including Trogonidae and Galbulae) 124 C. Strigiformes 129 D. Caprimulgiformes 132 E. Apodiformes 134 F. Family Incertae Sedis Trochilidae 135 G. Order Incertae Sedis Bucerotiformes (Including Upupae) 136 H. Piciformes 138 I. Passeriformes 139 X. The Water Bird Assemblage 141 A. Gruiformes 142 B. Family Incertae Sedis Ardeidae 165 79 Avian Biology, Vol. Vlll ISBN 0-12-249408-3 80 STORES L. OLSON C. Family Incertae Sedis Podicipedidae 168 D. Charadriiformes 169 E. Anseriformes 186 F. Ciconiiformes 188 G. Pelecaniformes 192 H. Procellariiformes 208 I. Gaviiformes 212 J. Sphenisciformes 217 XI. Conclusion 217 References 218 I. Introduction Avian paleontology has long been a poor stepsister to its mammalian counterpart, a fact that may be attributed in some measure to an insufRcien- cy of qualified workers and to the absence in birds of heterodont teeth, on which the greater proportion of the fossil record of mammals is founded. -

A Troodontid Dinosaur from the Latest Cretaceous of India
ARTICLE Received 14 Dec 2012 | Accepted 7 Mar 2013 | Published 16 Apr 2013 DOI: 10.1038/ncomms2716 A troodontid dinosaur from the latest Cretaceous of India A. Goswami1,2, G.V.R. Prasad3, O. Verma4, J.J. Flynn5 & R.B.J. Benson6 Troodontid dinosaurs share a close ancestry with birds and were distributed widely across Laurasia during the Cretaceous. Hundreds of occurrences of troodontid bones, and their highly distinctive teeth, are known from North America, Europe and Asia. Thus far, however, they remain unknown from Gondwanan landmasses. Here we report the discovery of a troodontid tooth from the uppermost Cretaceous Kallamedu Formation in the Cauvery Basin of South India. This is the first Gondwanan record for troodontids, extending their geographic range by nearly 10,000 km, and representing the first confirmed non-avian tetanuran dinosaur from the Indian subcontinent. This small-bodied maniraptoran dinosaur is an unexpected and distinctly ‘Laurasian’ component of an otherwise typical ‘Gondwanan’ tetrapod assemblage, including notosuchian crocodiles, abelisauroid dinosaurs and gondwanathere mammals. This discovery raises the question of whether troodontids dispersed to India from Laurasia in the Late Cretaceous, or whether a broader Gondwanan distribution of troodontids remains to be discovered. 1 Department of Genetics, Evolution, and Environment, University College London, London WC1E 6BT, UK. 2 Department of Earth Sciences, University College London, London WC1E 6BT, UK. 3 Department of Geology, Centre for Advanced Studies, University of Delhi, New Delhi 110 007, India. 4 Geology Discipline Group, School of Sciences, Indira Gandhi National Open University, New Delhi 110 068, India. 5 Division of Paleontology and Richard Gilder Graduate School, American Museum of Natural History, New York, New York 10024, USA. -

Bird Observer
Bird Observer VOLUME 39, NUMBER 2 APRIL 2011 HOT BIRDS On November 20 the Hampshire Bird Club was waiting at Quabbin headquarters for the rest of the group to arrive when Larry Therrien spotted a flock of 19 swans in the distance— Tundra Swans! Ian Davies took this photograph (left). Since 2003 Cave Swallows have been a specialty of November, showing up in coastal locations in increasing numbers over the years. This year there was a flurry of reports along the New England coast. On Thanksgiving Day, Margo Goetschkes took this photograph (right) of one of the birds at Salisbury. On November 30, Vern Laux got a call from a contractor reporting a “funny bird” at the Nantucket dump. Vern hustled over and was rewarded with great views of this Fork-tailed Flycatcher (left). Imagine: you’re photographing a Rough- legged Hawk in flight, and all of a sudden it is being mobbed—by a Northern Lapwing (right)! That’s what happened to Jim Hully on December 2 on Plum Island. This is only the second state record for this species, the first being in Chilmark in December of 1996. On April 9, Keelin Miller found an interesting gull at Kalmus Beach in Hyannis. As photographs were circulated, opinions shifted toward a Yellow-legged Gull (left). Check out Jeremiah Trimble’s photo from April 13. CONTENTS BIRDING THE LAKEVILLE PONDS OF PLYMOUTH COUNTY, MASSACHUSETTS Jim Sweeney 73 THE FINAL YEAR OF THE BREEDING BIRD ATLAS: GOING OVER THE TOP John Galluzzo 83 37 YEARS OF NIGHTHAWKING Tom Gagnon 86 LEIF J ROBINSON: MAY 21, 1939 – FEBRUARY 28, 2011 Soheil Zendeh 93 FIELD NOTES Double-crested Cormorant Has Trouble Eating a Walking Catfish William E. -

Appendix A. Supplementary Material
Appendix A. Supplementary material Comprehensive taxon sampling and vetted fossils help clarify the time tree of shorebirds (Aves, Charadriiformes) David Cernˇ y´ 1,* & Rossy Natale2 1Department of the Geophysical Sciences, University of Chicago, Chicago 60637, USA 2Department of Organismal Biology & Anatomy, University of Chicago, Chicago 60637, USA *Corresponding Author. Email: [email protected] Contents 1 Fossil Calibrations 2 1.1 Calibrations used . .2 1.2 Rejected calibrations . 22 2 Outgroup sequences 30 2.1 Neornithine outgroups . 33 2.2 Non-neornithine outgroups . 39 3 Supplementary Methods 72 4 Supplementary Figures and Tables 74 5 Image Credits 91 References 99 1 1 Fossil Calibrations 1.1 Calibrations used Calibration 1 Node calibrated. MRCA of Uria aalge and Uria lomvia. Fossil taxon. Uria lomvia (Linnaeus, 1758). Specimen. CASG 71892 (referred specimen; Olson, 2013), California Academy of Sciences, San Francisco, CA, USA. Lower bound. 2.58 Ma. Phylogenetic justification. As in Smith (2015). Age justification. The status of CASG 71892 as the oldest known record of either of the two spp. of Uria was recently confirmed by the review of Watanabe et al. (2016). The younger of the two marine transgressions at the Tolstoi Point corresponds to the Bigbendian transgression (Olson, 2013), which contains the Gauss-Matuyama magnetostratigraphic boundary (Kaufman and Brigham-Grette, 1993). Attempts to date this reversal have been recently reviewed by Ohno et al. (2012); Singer (2014), and Head (2019). In particular, Deino et al. (2006) were able to tightly bracket the age of the reversal using high-precision 40Ar/39Ar dating of two tuffs in normally and reversely magnetized lacustrine sediments from Kenya, obtaining a value of 2.589 ± 0.003 Ma. -

A Bird's Eye View of the Evolution of Avialan Flight
Chapter 12 Navigating Functional Landscapes: A Bird’s Eye View of the Evolution of Avialan Flight HANS C.E. LARSSON,1 T. ALEXANDER DECECCHI,2 MICHAEL B. HABIB3 ABSTRACT One of the major challenges in attempting to parse the ecological setting for the origin of flight in Pennaraptora is determining the minimal fluid and solid biomechanical limits of gliding and powered flight present in extant forms and how these minima can be inferred from the fossil record. This is most evident when we consider the fact that the flight apparatus in extant birds is a highly integrated system with redundancies and safety factors to permit robust performance even if one or more components of their flight system are outside their optimal range. These subsystem outliers may be due to other adaptive roles, ontogenetic trajectories, or injuries that are accommodated by a robust flight system. This means that many metrics commonly used to evaluate flight ability in extant birds are likely not going to be precise in delineating flight style, ability, and usage when applied to transitional taxa. Here we build upon existing work to create a functional landscape for flight behavior based on extant observations. The functional landscape is like an evolutionary adap- tive landscape in predicting where estimated biomechanically relevant values produce functional repertoires on the landscape. The landscape provides a quantitative evaluation of biomechanical optima, thus facilitating the testing of hypotheses for the origins of complex biomechanical func- tions. Here we develop this model to explore the functional capabilities of the earliest known avialans and their sister taxa. -

Late Pleistocene Birds from Kingston Saltpeter Cave, Southern Appalachian Mountains, Georgia
Bull. Fla. Mus. Nat. Hist. (2005) 45(4):231-248 231 LATE PLEISTOCENE BIRDS FROM KINGSTON SALTPETER CAVE, SOUTHERN APPALACHIAN MOUNTAINS, GEORGIA David W. Steadman1 Kingston Saltpeter Cave, Bartow County, Georgia, has produced late Quaternary fossils of 38 taxa of birds. The presence of extinct species of mammals, and three radiocarbon dates on mammalian bone collagen ranging from approximately 15,000 to 12,000 Cal B.P., indicate a late Pleistocene age for this fauna, although a small portion of the fossils may be Holocene in age. The birds are dominated by forest or woodland species, especially the Ruffed Grouse (Bonasa umbellus) and Passenger Pigeon (Ectopistes migratorius). Species indicative of brushy or edge habitats, wetlands, and grasslands also are present. They include the Greater Prairie Chicken (Tympanuchus cupido, a grassland indicator) and Black-billed Magpie (Pica pica, characteristic of woody edges bordering grasslands). Both of these species reside today no closer than 1000+ km to the west (mainly northwest) of Georgia. An enigmatic owl, perhaps extinct and undescribed, is represented by two juvenile tarsometatarsi. The avifauna of Kingston Saltpeter Cave suggests that deciduous or mixed deciduous/coniferous forests and woodlands were the dominant habitats in the late Pleistocene of southernmost Appalachia, with wetlands and grasslands present as well. Key Words: Georgia; late Pleistocene; southern Appalachians; Aves; extralocal species; faunal change INTRODUCTION dates have been determined from mammal bones at Numerous late Pleistocene vertebrate faunas have been KSC. The first (10,300 ± 130 yr B.P.; Beta-12771, un- recovered from caves and other karst features of the corrected for 13C/12C; = 12,850 – 11,350 Cal B.P.) is Appalachian region (listed in Lundelius et al. -
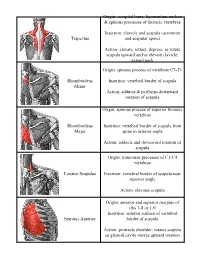
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Coracoid Process Anatomy: a Cadaveric Study of Surgically Relevant Structures Jorge Chahla, M.D., Ph.D., Daniel Cole Marchetti, B.A., Gilbert Moatshe, M.D., Márcio B
Quantitative Assessment of the Coracoacromial and the Coracoclavicular Ligaments With 3-Dimensional Mapping of the Coracoid Process Anatomy: A Cadaveric Study of Surgically Relevant Structures Jorge Chahla, M.D., Ph.D., Daniel Cole Marchetti, B.A., Gilbert Moatshe, M.D., Márcio B. Ferrari, M.D., George Sanchez, B.S., Alex W. Brady, M.Sc., Jonas Pogorzelski, M.D., M.H.B.A., George F. Lebus, M.D., Peter J. Millett, M.D., M.Sc., Robert F. LaPrade, M.D., Ph.D., and CAPT Matthew T. Provencher, M.D., M.C., U.S.N.R. Purpose: To perform a quantitative anatomic evaluation of the (1) coracoid process, specifically the attachment sites of the conjoint tendon, the pectoralis minor, the coracoacromial ligament (CAL), and the coracoclavicular (CC) ligaments in relation to pertinent osseous and soft tissue landmarks; (2) CC ligaments’ attachments on the clavicle; and (3) CAL attachment on the acromion in relation to surgically relevant anatomic landmarks to assist in planning of the Latarjet procedure, acromioclavicular (AC) joint reconstructions, and CAL resection distances avoiding iatrogenic injury to sur- rounding structures. Methods: Ten nonpaired fresh-frozen human cadaveric shoulders (mean age 52 years, range 33- 64 years) were included in this study. A 3-dimensional coordinate measuring device was used to quantify the location of pertinent bony landmarks and soft tissue attachment areas. The ligament and tendon attachment perimeters and center points on the coracoid, clavicle, and acromion were identified and subsequently dissected off the bone. Coordinates of points along the perimeters of attachment sites were used to calculate areas, whereas coordinates of center points were used to determine distances between surgically relevant attachment sites and pertinent bony landmarks.