Neurons Stimulation of Dorsal Root Ganglion Painful Pathways Induced
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nervous Tissue
Nervous Tissue • Controls and integrates all body activities within limits that maintain life • Three basic functions – sensing changes with sensory receptors • fullness of stomach or sun on your face – interpreting and remembering those changes – reacting to those changes with effectors • muscular contractions • glandular secretions Major Structures of the Nervous System • Brain, cranial nerves, spinal cord, spinal nerves, ganglia, enteric plexuses and sensory receptors Organization of the Nervous System • CNS is brain and spinal cord • PNS is everything else Nervous System Divisions • Central nervous system (CNS) – consists of the brain and spinal cord • Peripheral nervous system (PNS) – consists of cranial and spinal nerves that contain both sensory and motor fibers – connects CNS to muscles, glands & all sensory receptors Subdivisions of the PNS • Somatic (voluntary) nervous system (SNS) – neurons from cutaneous and special sensory receptors to the CNS – motor neurons to skeletal muscle tissue • Autonomic (involuntary) nervous systems – sensory neurons from visceral organs to CNS – motor neurons to smooth & cardiac muscle and glands • sympathetic division (speeds up heart rate) • parasympathetic division (slow down heart rate) • Enteric nervous system (ENS) – involuntary sensory & motor neurons control GI tract – neurons function independently of ANS & CNS Neurons • Functional unit of nervous system • Have capacity to produce action potentials – electrical excitability • Cell body – single nucleus with prominent nucleolus – Nissl -

Canine Dorsal Root Ganglia Satellite Glial Cells Represent an Exceptional Cell Population with Astrocytic and Oligodendrocytic P
www.nature.com/scientificreports OPEN Canine dorsal root ganglia satellite glial cells represent an exceptional cell population with astrocytic and Received: 17 August 2017 Accepted: 6 October 2017 oligodendrocytic properties Published: xx xx xxxx W. Tongtako1,2, A. Lehmbecker1, Y. Wang1,2, K. Hahn1,2, W. Baumgärtner1,2 & I. Gerhauser 1 Dogs can be used as a translational animal model to close the gap between basic discoveries in rodents and clinical trials in humans. The present study compared the species-specifc properties of satellite glial cells (SGCs) of canine and murine dorsal root ganglia (DRG) in situ and in vitro using light microscopy, electron microscopy, and immunostainings. The in situ expression of CNPase, GFAP, and glutamine synthetase (GS) has also been investigated in simian SGCs. In situ, most canine SGCs (>80%) expressed the neural progenitor cell markers nestin and Sox2. CNPase and GFAP were found in most canine and simian but not murine SGCs. GS was detected in 94% of simian and 71% of murine SGCs, whereas only 44% of canine SGCs expressed GS. In vitro, most canine (>84%) and murine (>96%) SGCs expressed CNPase, whereas GFAP expression was diferentially afected by culture conditions and varied between 10% and 40%. However, GFAP expression was induced by bone morphogenetic protein 4 in SGCs of both species. Interestingly, canine SGCs also stimulated neurite formation of DRG neurons. These fndings indicate that SGCs represent an exceptional, intermediate glial cell population with phenotypical characteristics of oligodendrocytes and astrocytes and might possess intrinsic regenerative capabilities in vivo. Since the discovery of glial cells over a century ago, substantial progress has been made in understanding the origin, development, and function of the diferent types of glial cells in the central nervous system (CNS) and peripheral nervous system (PNS)1. -

Fundamentals of Nervous System and Nervous Tissue
Fundamentals of Nervous System and Nervous Tissue Chapter 12 Nervous System The nervous system is the main system to communicate and coordinate body activities by sending electrical impulses. Nervous system forms a communication network in whole body. Endocrine system communicates through chemical messengers – hormones. 12 pairs of Cranial nerves arise from brain Brain (Part of PNS) Central NS 31 pairs of spinal nerves arise from spinal Spinal nerve cord nerve cord (Part of PNS) Somatic sensory Afferent Division Visceral sensory Peripheral NS Somatic NS Efferent Division Sympathetic Autonomic NS Parasympathetic Neuron A neuron has a cell body. Many smaller branched appendages are called Dendrites. Dendrites bring in information (nerve impulse) to the cell body. A single longer appendage is called Axon. It takes information away from cell body. It branches at the end into terminal knobs. A terminal knob secretes a chemical called Neurotransmitter in the gap to the next neuron or muscle membrane. 3-types of neurons (on basis of function) Specialized nerve cells are called Neurons. Sensory neurons bring information from sense organs like eyes to CNS. Sensory = Affrent. Somatic Sensory = coming from body wall - skin, muscles and joints; Visceral Sensroy = coming from internal organs - viscera Motor neurons take information from CNS to effectors like muscles or glands. Motor = Effrent. Somatic Motor – going to skeletal muscles and Visceral Motor – going to smooth or cardiac muscles. Inter-neurons receive information from sensory neurons and -

Gamma Motor Neurons Survive and Exacerbate Alpha Motor Neuron Degeneration In
Gamma motor neurons survive and exacerbate alpha PNAS PLUS motor neuron degeneration in ALS Melanie Lalancette-Heberta,b, Aarti Sharmaa,b, Alexander K. Lyashchenkoa,b, and Neil A. Shneidera,b,1 aCenter for Motor Neuron Biology and Disease, Columbia University, New York, NY 10032; and bDepartment of Neurology, Columbia University, New York, NY 10032 Edited by Rob Brownstone, University College London, London, United Kingdom, and accepted by Editorial Board Member Fred H. Gage October 27, 2016 (received for review April 4, 2016) The molecular and cellular basis of selective motor neuron (MN) the muscle spindle and control the sensitivity of spindle afferent vulnerability in amyotrophic lateral sclerosis (ALS) is not known. In discharge (15); beta (β) skeletofusimotor neurons innervate both genetically distinct mouse models of familial ALS expressing intra- and extrafusal muscle (16). In addition to morphological mutant superoxide dismutase-1 (SOD1), TAR DNA-binding protein differences, distinct muscle targets, and the absence of primary 43 (TDP-43), and fused in sarcoma (FUS), we demonstrate selective afferent (IA)inputsonγ-MNs, these functional MN subtypes also degeneration of alpha MNs (α-MNs) and complete sparing of differ in their trophic requirements, and γ-MNs express high levels gamma MNs (γ-MNs), which selectively innervate muscle spindles. of the glial cell line-derived neurotropic factor (GDNF) receptor Resistant γ-MNs are distinct from vulnerable α-MNs in that they Gfrα1 (17). γ-MNs are also molecularly distinguished by the ex- lack synaptic contacts from primary afferent (IA) fibers. Elimination pression of other selective markers including the transcription α of these synapses protects -MNs in the SOD1 mutant, implicating factor Err3 (18), Wnt7A (19), the serotonin receptor 1d (5-ht1d) this excitatory input in MN degeneration. -

11 Introduction to the Nervous System and Nervous Tissue
11 Introduction to the Nervous System and Nervous Tissue ou can’t turn on the television or radio, much less go online, without seeing some- 11.1 Overview of the Nervous thing to remind you of the nervous system. From advertisements for medications System 381 Yto treat depression and other psychiatric conditions to stories about celebrities and 11.2 Nervous Tissue 384 their battles with illegal drugs, information about the nervous system is everywhere in 11.3 Electrophysiology our popular culture. And there is good reason for this—the nervous system controls our of Neurons 393 perception and experience of the world. In addition, it directs voluntary movement, and 11.4 Neuronal Synapses 406 is the seat of our consciousness, personality, and learning and memory. Along with the 11.5 Neurotransmitters 413 endocrine system, the nervous system regulates many aspects of homeostasis, including 11.6 Functional Groups respiratory rate, blood pressure, body temperature, the sleep/wake cycle, and blood pH. of Neurons 417 In this chapter we introduce the multitasking nervous system and its basic functions and divisions. We then examine the structure and physiology of the main tissue of the nervous system: nervous tissue. As you read, notice that many of the same principles you discovered in the muscle tissue chapter (see Chapter 10) apply here as well. MODULE 11.1 Overview of the Nervous System Learning Outcomes 1. Describe the major functions of the nervous system. 2. Describe the structures and basic functions of each organ of the central and peripheral nervous systems. 3. Explain the major differences between the two functional divisions of the peripheral nervous system. -

Dorsal Root Injury—A Model for Exploring Pathophysiology and Therapeutic Strategies in Spinal Cord Injury
cells Review Dorsal Root Injury—A Model for Exploring Pathophysiology and Therapeutic Strategies in Spinal Cord Injury Håkan Aldskogius * and Elena N. Kozlova Laboratory of Regenertive Neurobiology, Biomedical Center, Department of Neuroscience, Uppsala University, 75124 Uppsala, Sweden; [email protected] * Correspondence: [email protected] Abstract: Unraveling the cellular and molecular mechanisms of spinal cord injury is fundamental for our possibility to develop successful therapeutic approaches. These approaches need to address the issues of the emergence of a non-permissive environment for axonal growth in the spinal cord, in combination with a failure of injured neurons to mount an effective regeneration program. Experimental in vivo models are of critical importance for exploring the potential clinical relevance of mechanistic findings and therapeutic innovations. However, the highly complex organization of the spinal cord, comprising multiple types of neurons, which form local neural networks, as well as short and long-ranging ascending or descending pathways, complicates detailed dissection of mechanistic processes, as well as identification/verification of therapeutic targets. Inducing different types of dorsal root injury at specific proximo-distal locations provide opportunities to distinguish key components underlying spinal cord regeneration failure. Crushing or cutting the dorsal root allows detailed analysis of the regeneration program of the sensory neurons, as well as of the glial response at the dorsal root-spinal cord interface without direct trauma to the spinal cord. At the same time, a lesion at this interface creates a localized injury of the spinal cord itself, but with an initial Citation: Aldskogius, H.; Kozlova, neuronal injury affecting only the axons of dorsal root ganglion neurons, and still a glial cell response E.N. -

The “Road Map”
PRACTICAL ROADMAP NERVOUS TISSUE DR N GRAVETT NEURONS • MOTOR • SENSORY Anterior (ventral) horn Dorsal root of spinal of spinal cord cord Multipolar Pseudounipolar ANTERIOR HORN CELLS • Slide 64 Spinal Cord (vervet monkey) Stain: Kluver and Berrera Technique NOTE: with this technique, myelin stains dark blue and basophilic substances such as rER and nuclei stain violet. In this case we use “blue” and “purple” to describe the staining and not eosinophilic and basophilic. SPINAL CORD Anterior Ventral Horn Arachnoid Ventricle Pia Mater Grey Matter White Matter Posterior Horn Dura Mater Dorsal ANTERIOR HORN CELL Neuropil Cell Body Dendrite Vesicular Nucleus Nucleolus Nucleus of Nissl Bodies Neuroglial Cell ANTERIOR HORN CELL Neuropil Cell Body Vesicular Nucleus Nucleolus Nissl Body Nucleus of Neuroglial Cell Dendrite Nissl Body Axon Hillock Axon SPINAL (DORSAL ROOT) GANGLION CELLS • Slide 62 Spinal Ganglion Stain: H&E NOTE: The spinal ganglion is also known as the dorsal root ganglia and contains pseudounipolar neuron cell bodies. SPINAL (DORSAL ROOT) GANGLIA Cell Bodies Processes (Axons and Dendrites) SPINAL (DORSAL ROOT) GANGLIA Cell Bodies Processes (Axons and Dendrites) NOTE: The neuronal cell bodies of the dorsal root ganglia are “clumped” together, and one cannot see any processes entering or leaving the cell bodies. The processes (axons and dendrites) are seen towards the edge/periphery of the group of cell bodies. SPINAL (DORSAL ROOT) GANGLIA Satellite cells (arranged in ring like fashion around the cell body) Cell Body Nucleolus Vesicular Fine Granular Nucleus Nissl Substance Nucleus of Satellite cell PERIPHERAL BRANCH OF A SPINAL NERVE • Slide 32 Median Nerve Stain: Mallory’s Technique NOTE: Three dyes are used in Mallory’s technique, which results in collagen fibres (such as connective tissue) staining blue, the “neurokeratin” staining red, and nuclei staining reddish-orange PERIPHERAL NERVE Myelinated Axons Vein L.S. -

Cells of the Nervous System Two Major Cell/Tissue Types in Nervous System
Cells of the Nervous System two major cell/tissue types in Nervous System: neurons – impulse conduction 100 Billion generally no mitosis neuroglia – support, protection, insulation, etc [need specialized cells because of unique sensitivity of neurons to their environment] 900 Billion some mitosis Neuroglia 1. astrocytes 2. oligodendroglia 3. microglia 4. ependymal cells 5. Schwann cells 1. Astrocytes largest and most abundant type form tight webs around brains capillaries =blood/brain barrier small molecules (O2, CO2, alcohol) diffuse rapidly larger molecules penetrate slowly or not at all this blockage of free exchange between capillaries and tissues is unique for nervous tissue => prevents sudden and extreme fluctuations in composition of tissue fluid in CNS => protects irreplaceable neurons from damage 2. Oligodendrocytes (oligodendroglia) smaller cells, fewer processes clustered around nerve cell bodies help hold nerve fibers together produce myelin sheath (electrical insulation) around neurons in CNS [myelin=fatty substance] 3. Microglia small stationary cells in inflamed or degenerating brain tissue they: enlarge move about 1 carry out phagocytosis of microbes and cellular debris 4. Ependymal Cells ciliated cells line ventricles and spinal canal help to circulate CerebroSpinal Fluid 5. Schwann Cells found only in PNS form a segmental wrapping around nerve fibers each segment is produced by 1 Schwann cell gaps between cells = Nodes of Ranvier form neurilemma and myelin sheath in PNS neurons myelin (in CNS and PNS) can be: thick = -

29 1. ABSTRACT 2. INTRODUCTION Oligodendrocyte Progenitor Cells
[Frontiers in Bioscience, Scholar, 8, 29-43, January 1, 2016] Oligodendrocyte progenitor cells: the ever mitotic cells of the CNS Bjoern Neumann1, Ilias Kazanis1,2 1Wellcome Trust- MRC Cambridge Stem Cell Institute and Department of Clinical Neurosciences, University of Cambridge, UK, 2Lab of Developmental Biology, Department of Biology, University of Patras, Patras, Greece TABLE OF CONTENTS 1. Abstract 2. Introduction 3. OPCs remain mitotically active throughout the lifespan of the organism 3.1. Development 3.2. Adult brain homeostasis and pathology 4. Mechanisms of control of mitosis in OPCs 5. Acknowledgments 6. References 1. ABSTRACT Oligodendrocyte Progenitor Cells (OPCs) first exited the cell cycle in order to terminally differentiate into appear at mid embryogenic stages during development mature OLs. However, a subpopulation of OPCs survives of the mammalian CNS and a mitotically active population for the life span of the organism (both in humans and of them remains present even into late adulthood. During rodents) as adult OPCs scattered throughout the grey the life-time of the organism they initially proliferate and and white matter where it represents approximately migrate in order to populate the whole nervous tissue, 5% of all cells (2) (Figure 1). Recent experimental work then they massively generate oligodendrocytesand finally in the mouse has revealed that adult OPCs probably they switch to a less mitotically active phase generating sustain myelin homeostasis by continuing to proliferate new oligodendrocytes at a slow rate in the adult brain; and to generate new OLs in the adult brain (3-5); importantly, they can regenerate acutely or chronically albeit exhibiting a gradually slower cell cycle and with destroyed myelin. -

Oligodendrocytes and CNS Myelin Are Nonpermissive Substrates for Neurite Growth and Fibroblast Spreading in Vi&O
The Journal of Neuroscience, July 1988, 8(7): 2381-2393 Oligodendrocytes and CNS Myelin Are Nonpermissive Substrates for Neurite Growth and Fibroblast Spreading in vi&o Martin E. Schwab and Pica Caroni Brain Research Institute of the University of Zurich, CH-8029 Zurich, Switzerland To study the interaction of neurons with CNS glial cells, (Tello, 1911; Ramon y Cajal, 1928; Benfey and Aguayo, 1982; dissociated sympathetic or sensory ganglion cells or fetal Richardson et al., 1984; So and Aguayo, 1985). These studies retinal cells were plated onto cultures of dissociated optic assigneda crucial role to the microenvironment of the growing nerve glial cells of young rats. Whereas astrocytes favored fibers, whereby peripheral nerve tissue should allow, support, neuron adhesion and neurite outgrowth, oligodendrocytes or provoke neurite regeneration. The involvement of neuro- differed markedly in their properties as neuronal substrates. trophic and neurotropic factors-produced by Schwann cellsbut Immature (O,+, A,B,+, GalCm) oligodendrocytes were fre- not by CNS glia-was suggested60 years ago by Ramon y Cajal quently contacted by neurons and neurites. In contrast, dif- (1928). In fact, a marked increase in the production of neuro- ferentiated oligodendrocytes (O,+, A$-, GalC+) represented trophic factors and cell adhesionmolecules by Schwann cells in a nonpermissive substrate for neuronal adhesion and neurite responseto denervation has recently been observed (Richardson growth. When neuroblastoma cells or 3T3 fibroblasts were and Ebendal, 1982; Longo et al., 1984; Abrahamson et al., 1986; plated into optic nerve glial cultures, the same differences Daniloff et al., 1986). However, neurotrophic factors are also were observed; differentiated oligodendrocytes were non- presentin developing and adult CNS, and increasedneurotroph- permissive for cell adhesion, neurite growth, or fibroblast ic activities were found at sites of CNS lesions (Barde et al., spreading. -

A Light and Electron Microscope Study of Long
A LIGHT AND ELECTRON MICROSCOPE STUDY OF LONG-TERM ORGANIZED CULTURES OF RAT DORSAL ROOT GANGLIA MARY BARTLETT BUNGE, RICHARD P. BUNGE, EDITH R. PETERSON, and MARGARET R. MURRAY From the Departments of Anatomy and Surgery, Columbia University College of Physicians and Downloaded from http://rupress.org/jcb/article-pdf/32/2/439/1263268/439.pdf by guest on 01 October 2021 Surgeons, New York ABSTRACT Dorsal root ganglia from fetal rats were explanted on collagen-coated coverslips and carried in Maximow double-coverslip assemblies for periods up to 3 months. These cultured ganglia were studied in the living state, in stained whole mounts, and in sections after OsO4 fixation and Epon embedment. From the central cluster of nerve cell bodies, neurites emerge to form a rich network of fascicles which often reach the edge of the carrying coverslip. The neurons resemble their in vivo counterparts in nuclear and cytoplasmic content and organization; e.g., they appear as "light" or "dark" cells, depending on the amount of cytoplasmic neuro- filaments. Satellite cells form a complete investment around the neuronal soma and are themselves everywhere covered by basement membrane. The neuron-satellite cell boundary is complicated by spinelike processes arising from the neuronal soma. Neuron size, myelin- ated fiber diameter, and internode length in the cultures do not reach the larger of the values known for ganglion and peripheral nerve in situ (30). Unmyelinated and myelinated nerve fibers and associated Schwann cells and endoneurial and perineurial components are orga- nized into typical fascicles. The relationship of the Schwann cell and its single myelinated fiber or numerous unmyelinated fibers and the properties of myelin, such as lamellar spacing, mesaxons, Schmidt-Lanterman clefts, nodes of Ranvier, and protuberances, mimic the in vivo pattern. -
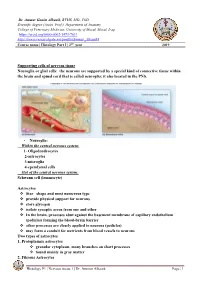
Supporting Cells of Nervous Tissue Neuroglia Or Glial Cells
Dr. Ammar Ganim Alhaaik, BVMS, MSc, PhD Scientific degree (Assist. Prof.), Department of Anatomy College of Veterinary Medicine, University of Mosul, Mosul, Iraq https://orcid.org/0000-0002-1473-7631 https://www.researchgate.net/profile/Ammar_Alhaaik4 Course name | Histology Part I | 2nd year 2019 Supporting cells of nervous tissue Neuroglia or glial cells: the neurons are supported by a special kind of connective tissue within the brain and spinal cord that is called neuroglia; it also located in the PNS. • Neuroglia: Within the central nervous system: 1- Oligodendrocytes 2-astrocytes 3-microglia 4-ependymal cells Out of the central nervous system: Schwann cell (lemmocyte) Astrocytes ❖ Star shape and most numerous type ❖ provide physical support for neurons ❖ store glycogen ❖ isolate synaptic areas from one and other ❖ In the brain, processes abut against the basement membrane of capillary endothelium (pedicles) forming the blood-brain barrier ❖ other processes are closely applied to neurons (pedicles) ❖ may form a conduit for nutrients from blood vessels to neurons Two types of astrocytes 1. Protoplasmic astrocytes ❖ granular cytoplasm, many branches on short processes ❖ found mainly in gray matter 2. Fibrous Astrocytes Histology P1 | Nervous tissue 1 | Dr. Ammar Alhaaik Page | 1 ❖ have longer slender processes ❖ found mainly in white matter (but also occur in gray matter). Ependymal cells ❖ ciliated cells forming single layer of simple cuboidal to low columnar epithelium that lines the entire neurocoel ❖ Epithelial cells that line ventricles and central cavities of brain and spinal cord-secrete CSF ❖ ciliary action acts to circulate cerebral spinal fluid. The Synapse Concept: Synapses are highly specialized intercellular junctions which link the neurons of each nervous pathway.