Level 3 Organic Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nomenclature of Carboxylic Acid Derivatives Acid Halide Substituents
Gentilucci, Carboxylic Acid Derivatives Nomenclature of Carboxylic Acid Derivatives Gentilucci, Carboxylic Acid Derivatives Acid halides 1. Alkane + the suffix -oyl followed by the halogen. 2. Select the longest continuous carbon chain, containing the acyl group. 3. Number the carbon chain, beginning at the end nearest to the acyl group. 4. Number the substituents and write the name, listing substituents alphabetically. Acid halide substituents attached to rings are named using the suffix - carbonyl. 1 Gentilucci, Carboxylic Acid Derivatives Anhydrides 1. Symmetrical: replace the ending "acid" with "anhydride ". 2. Asymmetrical: select the longest continuous carbon chain, containing the carboxylic acid group, and derive the parent name by replacing the -e ending with -oic anhydride . 3. Number the carbon chain, beginning at the end nearest to the acyl group. 4. Number the substituents and write the name, listing substituents alphabetically. Gentilucci, Carboxylic Acid Derivatives Amides are named by replacing the ending -oic acid with -amide . 1. Select the longest continuous carbon chain, containing the acyl group, and derive the parent name by replacing the -e ending with -amide . 2. Number the carbon chain, beginning at the end nearest to the acyl group. 3. Number the substituents and write the name, listing substituents alphabetically. 4. If the nitrogen atom is further substituted, the substituents are preceded by N- to indicate that they are attached to the nitrogen. Acid halide substituents attached to rings are named using the suffix - carboxamide. 2 Gentilucci, Carboxylic Acid Derivatives Carboxylate esters 1. Select the longest continuous carbon chain containing the acyl group, and derive the parent name by replacing the -e ending with –oate . -

Carbonyl Compounds
Carbonyl Compounds What are Carbonyl Compounds? Carbonyl compounds are compounds that contain the C=O (carbonyl) group. Preparation of Aldehydes: 1. Preparation from Acid Chloride (Rosenmund Reduction): This reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918. The reaction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction, a hydrogenolysis, is catalysed by palladium on barium sulfate, which is sometimes called the Rosenmund catalyst. 2. Preparation from Nitriles: This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R- CN) using SnCl2 and HCl and quenching the resulting iminium salt ([R- + − CH=NH2] Cl ) with water (H2O). During the synthesis, ammonium chloride is also produced. The reaction is known as Stephen Aldehyde synthesis. Dr. Sumi Ganguly Page 1 3. Preparation from Grignard Reagent: When Grignard Reagent is reacted with HCN followed by hydrolysis aldehyde is produced. Preparation of Ketones: 1. Preparation from Acid Chloride (Friedel-Crafts Acylation): Acid chlorides when reacted with benzene in presence of anhydrous AlCl3, aromatic ketone are produced. However, only aromatic ketones can be prepared by following this method. In order to prepare both aromatic and aliphatic ketones acid chlorides is reacted with lithium dialkylcuprate (Gilman Reagnt). Dr. Sumi Ganguly Page 2 The lithium dialkyl cuprate is produced by the reaction of two equivalents of the organolithium reagent with copper (I) iodide. Example: 3. Preparation from Nitriles and Grignard Reagents: When Grignard Reagent is reacted with RCN followed by hydrolysis aldehyde is produced. Dr. Sumi Ganguly Page 3 Physical Characteristic of Carbonyl Compounds: 1) The boiling point of carbonyl compounds is higher than the alkanes with similar Mr. -

Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1
Chapter 7, Haloalkanes, Properties and Substitution Reactions of Haloalkanes Table of Contents 1. Alkyl Halides (Haloalkane) 2. Nucleophilic Substitution Reactions (SNX, X=1 or 2) 3. Nucleophiles (Acid-Base Chemistry, pka) 4. Leaving Groups (Acid-Base Chemistry, pka) 5. Kinetics of a Nucleophilic Substitution Reaction: An SN2 Reaction 6. A Mechanism for the SN2 Reaction 7. The Stereochemistry of SN2 Reactions 8. A Mechanism for the SN1 Reaction 9. Carbocations, SN1, E1 10. Stereochemistry of SN1 Reactions 11. Factors Affecting the Rates of SN1 and SN2 Reactions 12. --Eliminations, E1 and E2 13. E2 and E1 mechanisms and product predictions In this chapter we will consider: What groups can be replaced (i.e., substituted) or eliminated The various mechanisms by which such processes occur The conditions that can promote such reactions Alkyl Halides (Haloalkane) An alkyl halide has a halogen atom bonded to an sp3-hybridized (tetrahedral) carbon atom The carbon–chlorine and carbon– bromine bonds are polarized because the halogen is more electronegative than carbon The carbon-iodine bond do not have a per- manent dipole, the bond is easily polarizable Iodine is a good leaving group due to its polarizability, i.e. its ability to stabilize a charge due to its large atomic size Generally, a carbon-halogen bond is polar with a partial positive () charge on the carbon and partial negative () charge on the halogen C X X = Cl, Br, I Different Types of Organic Halides Alkyl halides (haloalkanes) sp3-hybridized Attached to Attached to Attached -

Indium Promoted-Convenient Method for Acylation of Alc이iols with Acyl Chlorides
Communications to the Editor Bull. Korean Chem. Soc. 2003, Vol. 24, No. 2 155 Indium Promoted-Convenient Method for Acylation of Alc이iols with Acyl Chlorides Dae Hyan Cho, Joong Gon Kim,f and Doo Ok Jang* Department of Chemistry, Yonsei University, Wonju 220-710, Korea ‘Biotechnology Division, Hanwha Chemical R & D Center, Daejeon 305-345, Korea Received November 9, 2002 Key Words : Indium, Alcohol, Acylation, Acyl chloride Even though various reagents for coupling of alcohols tions for the acylation of alcohols with acyl chlorides in the with carboxylic acids and transesterification of esters have presence of indium. The results are summarized in Table 1. been developed,1 there is still a great demand for a process Reaction of 2 (1 equiv) with 1 (1 equiv) in the presence of by using acyl chlorides for the acylation of alcohols in the indium (1 equiv) in CH3CN at room temperature produced case of substrates having steric hindrance or low reactivity. the corresponding ester in only 21% yield and the starting The acylation of alcohols with acyl chlorides is commonly acyl chloride and alcohol were recovered. The optimal yield carried out in the presence of tertiary amines such as 4- of the ester was attained with 3 equiv of 1 or 2 in the presence (methylamino) pyridine or 4 -pyrrolidinopyridine.2 Many of 3 equiv of indium. The solvent effect of acylation of 2 methods for the acylation of alcohols with acyl chlorides with 1 in the presence of indium was studied. The reaction have been developed using a variety of reagents.3 Most proceeded efficiently in common organic solvents such as recently, benzoylation of alcohols with lithium perchlorate DMF, Et2。,THF or CHzCh whereas non-polar solvents hase been reported.4 However, these methods have their own such as n-hexane or benzene gave poor yields of the ester. -

Part I. the Total Synthesis Of
AN ABSTRACT OF THE THESIS OF Lester Percy Joseph Burton forthe degree of Doctor of Philosophy in Chemistry presentedon March 20, 1981. Title: Part 1 - The Total Synthesis of (±)-Cinnamodialand Related Drimane Sesquiterpenes Part 2 - Photochemical Activation ofthe Carboxyl Group Via NAcy1-2-thionothiazolidines Abstract approved: Redacted for privacy DT. James D. White Part I A total synthesis of the insect antifeedant(±)-cinnamodial ( ) and of the related drimanesesquiterpenes (±)-isodrimenin (67) and (±)-fragrolide (72)are described from the diene diester 49. Hydro- boration of 49 provided the C-6oxygenation and the trans ring junction in the form of alcohol 61. To confirm the stereoselectivity of the hydroboration, 61 was convertedto both (t)-isodrimenin (67) and (±)-fragrolide (72) in 3 steps. A diisobutylaluminum hydride reduction of 61 followed by a pyridiniumchlorochromate oxidation and treatment with lead tetraacetate yielded the dihydrodiacetoxyfuran102. The base induced elimination of acetic acid preceded theepoxidation and provided 106 which contains the desired hydroxy dialdehydefunctionality of cinnamodial in a protected form. The epoxide 106 was opened with methanol to yield the acetal 112. Reduction, hydrolysis and acetylation of 112 yielded (t)- cinnamodial in 19% overall yield. Part II - Various N- acyl- 2- thionothiazolidineswere prepared and photo- lysed in the presence of ethanol to provide the corresponding ethyl esters. The photochemical activation of the carboxyl function via these derivatives appears, for practical purposes, to be restricted tocases where a-keto hydrogen abstraction and subsequent ketene formation is favored by acyl substitution. Part 1 The Total Synthesis of (±)-Cinnamodial and Related Drimane Sesquiterpenes. Part 2 Photochemical Activation of the Carboxyl Group via N-Acy1-2-thionothiazolidines. -

Classifying Halogenoalkanes Reactions Of
10 Halogenoalkanes H Naming Halogenoalkanes H H H H C H H H H Based on original alkane, with a prefix indicating halogen atom: H C C C H Fluoro for F; Chloro for Cl; Bromo for Br; Iodo for I. H C C C C H Br H H H Cl H H Substituents are listed alphabetically 1-bromopropane 2-chloro-2-methylbutane Classifying Halogenoalkanes Halogenoalkanes can be classified as primary, secondary or tertiary depending on the number of carbon atoms attached to the C-X functional group. H H H H H H H H C H H H H H C C C H H C C C H H C C C C H Br H H H Br H H Cl H H Primary halogenoalkane Secondary halogenoalkane Tertiary halogenoalkane One carbon attached to the Two carbons attached to the Three carbons attached to the carbon atom adjoining the carbon atom adjoining the carbon atom adjoining the halogen halogen halogen Reactions of Halogenoalkanes Halogenoalkanes undergo either substitution or elimination reactions Nucleophilic substitution reactions Substitution: swapping a halogen atom for another atom or groups of atoms - - Nucleophile: electron pair donator e.g. :OH , :NH3, CN :Nu represents any nucleophile – they The Mechanism: We draw (or outline) mechanisms to always have a lone pair and act as show in detail how a reaction proceeds electron pair donators Nu:- The nucleophiles The carbon has a small attack the positive H H H H positive charge because carbon atom of the electronegativity H C C X H C C + X- δ+ δ- Nu difference between the carbon and the halogen H H H H We use curly arrows in mechanisms (with A curly arrow will always two line heads) to show the movement of start from a of electrons or two electrons lone pair the centre of a bond The rate of these substitution reactions depends on the strength Bond enthalpy / of the C-X bond kJmol-1 The weaker the bond, the easier it is to break and the faster the reaction. -

Derivatives of Carboxylic Acids
Acylation A4 1 DERIVATIVES OF CARBOXYLIC ACIDS ACYL (ACID) CHLORIDES - RCOCl ACID ANHYDRIDES - (RCO)2O named from corresponding acid named from corresponding acid remove -ic add -yl chloride remove acid add anhydride CH3COCl ethanoyl chloride (CH3CO)2O ethanoic anhydride C6H5COCl benzene carbonyl chloride δ− δ+ O δ− CH C O 3 δ− δ+ O δ+ CH3 C δ− CH3 C δ− Cl O bonding in acyl chlorides bonding in acid anhydrides Chemical Properties • colourless liquids which fume in moist air • acyl chlorides are more reactive than anhydrides • attacked at the positive carbon centre by nucleophiles • nucleophiles include water, alcohols, ammonia and amines • undergo addition-elimination reactions Uses of Acylation Industrially Manufacture of Cellulose acetate - making fibres Aspirin (acetyl salicylic acid) - analgaesic Ethanoic anhydride is more useful • cheaper • less corrosive • less vulnerable to hydrolysis • less dangerous reaction Laboratory Used to make carboxylic acid, esters, amines, N-substituted amines Ethanoyl chloride is used as it • is more reactive • gives a cleaner reaction Q.1 Investigate how aspirin is made industrially and in the laboratory. Why are the reagents and chemicals different? What properties of Aspirin make it such a useful drug? 2 A4 Acylation ADDITION ELIMINATION REACTIONS - OVERVIEW Mechanism • species attacked by nucleophiles at the positive carbon end of the C=O bond • the nucleophile adds to the molecule • either Cl or RCOO¯ is eliminated • a proton is removed General example - with water ACID CHLORIDES H Cl + Cl H O O -
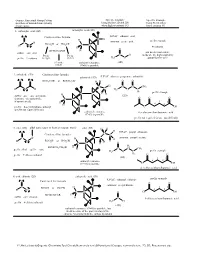
Generic Functional Group Pattern in Ordero of Nomenclature Priority in Our Course. Specific Example Using Suffix (2D and 3D)
Generic Functional Group Pattern Specific Example Specific Example in ordero of nomenclature priority Using Suffix (2D and 3D) Using Prefix when in our course. when highest priority FG lower priority FG 1. carboxylic acid (2D) carboxylic acid (3D) O Condensed line formula IUPAC: ethanoic acid O H H prefix example C H common: acetic acid RCO2H or HO2CR C C R O #-carboxy H O RCH(CO H)R' H suffix: -oic acid 2 O not used in our course FG on FG on C H (acids are the highest priority prefix: #-carboxy the right the left H3C O group that we use) FG in the carbonyl resonance (2D) middle (C=O) is possible 2. anhydride (2D) Condensed line formula anhydride (3D) IUPAC: ethanoic propanoic anhydride O O RCO2COR' or ROCO2CR' H H O O H C C C CH C 3 R O R H3C O C H H 2 prefix example H O C H suffix: -oic -oic anhydride (2D) (just one -oic anhydride, O O O C C O if symmetrical) 3 1 H 4 H 2 prefix: #-acyloxyalkanecarbonyl O O OH (prefix not required for us) carbonyl resonance 4-acyloxymethanebutanoic acid (C=O) is possible (prefix not required for us - too difficult) 3. ester (2D) alkyl name (goes in front as separate word) ester (3D) H H H IUPAC: propyl ethanoate O C Condensed line formula C O C common: propyl acetate C R' H H R O RCO2R' or R'O2CR H H C C H O H H2 RCH(CO2CH3)R' prefix: alkyl suffix: -oate H C C CH3 O H C O C prefix example 3 H prefix: #-alkoxycarbonyl 2 O O (2D) 3 1 carbonyl resonance 4 2 (C=O) is possible O OH 4-methoxycarbonylbutanoic acid 4. -

A Perovskite-Based Paper Microfluidic Sensor for Haloalkane Assays
ORIGINAL RESEARCH published: 26 April 2021 doi: 10.3389/fchem.2021.682006 A Perovskite-Based Paper Microfluidic Sensor for Haloalkane Assays Lili Xie 1, Jie Zan 1, Zhijian Yang 1, Qinxia Wu 1, Xiaofeng Chen 1, Xiangyu Ou 1, Caihou Lin 2*, Qiushui Chen 1,3* and Huanghao Yang 1,3* 1 Ministry of Education (MOE) Key Laboratory for Analytical Science of Food Safety and Biology, Fujian Provincial Key Laboratory of Analysis and Detection Technology for Food Safety, College of Chemistry, Fuzhou University, Fuzhou, China, 2 Department of Neurosurgery, Fujian Medical University Union Hospital, Fuzhou, China, 3 Fujian Science and Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, China Detection of haloalkanes is of great industrial and scientific importance because some Edited by: haloalkanes are found serious biological and atmospheric issues. The development Jinyi Lin, of a flexible, wearable sensing device for haloalkane assays is highly desired. Here, Nanjing Tech University, China we develop a paper-based microfluidic sensor to achieve low-cost, high-throughput, Reviewed by: Xiaobao Xu, and convenient detection of haloalkanes using perovskite nanocrystals as a nanoprobe Nanjing University of Science and through anion exchanging. We demonstrate that the CsPbX3 (X = Cl, Br, or I) Technology, China nanocrystals are selectively and sensitively in response to haloalkanes (CH2Cl2, CH2Br2), Xuewen Wang, Northwestern Polytechnical and their concentrations can be determined as a function of photoluminescence University, China spectral shifts of perovskite nanocrystals. In particular, an addition of nucleophilic trialkyl *Correspondence: phosphines (TOP) or a UV-photon-induced electron transfer from CsPbX3 nanocrystals is Caihou Lin [email protected] responsible for achieving fast sensing of haloalkanes. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

N Goalby Chemrevise.Org 1 Reaction with Water
Carboxylic acid derivatives: Acyl Chlorides and Acid Anhydrides Acyl Chlorides Acid Anhydrides Acid anhydrides have a similar reactivity to O Acyl chlorides are much O acyl chlorides and therefore bring about the CH3 C more reactive than same changes in functional groups. carboxylic acids CH3 C Cl O ethanoyl chloride The main difference is the by-products. CH3 C Acyl chlorides mostly give off HCl. Acid O anhydrides give off RCOOH ethanoic anhydride. Explaining reactivity Many of the reactions of the carboxylic acid derivatives follow the pattern below with an attack by an nucleophile. O O CH3 C + :X- CH3 C + :W- On a simplistic level, the stronger the electron attracting power of ‘W’, the W X more positive the carbon, and the more attractive the carbon is to Where –W: and :W- can be –Cl and Cl- (acyl chlorides) nucleophiles. - or -OCH3 and OCH3 (esters) The relative attractive powers of the –W: are - `or -NH2 and NH2 (amides) -Cl > -OH > -OCH3 > -NH2 Therefore in the case of hydrolysis reactions, acyl chlorides are highly reactive and will be hydrolysed by weak nucleophiles such as water. Amides and Esters contain only weak electron attracting W groups and need strong nucleophiles such as hydroxide ions in NaOH to hydrolyse. This difference in reactivity is caused by a combination of two factors 1. the electronegativity of the Cl’s, N’s and O’s causing electron density to be withdrawn from the carbon making the carbon more positive and more attractive to nucleophiles- This factor makes them more reactive. 2. delocalisation of the lone pairs on these atoms into the carbonyl system which reduces the reactivity.