Salinity Tolerances and Use of Saline Environments by Freshwater Turtles: Implications of Sea Level Rise
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laws of Malaysia
LAWS OF MALAYSIA ONLINE VERSION OF UPDATED TEXT OF REPRINT Act 716 WILDLIFE CONSERVATION ACT 2010 As at 1 December 2014 2 WILDLIFE CONSERVATION ACT 2010 Date of Royal Assent … … 21 October 2010 Date of publication in the Gazette … … … 4 November 2010 Latest amendment made by P.U.(A)108/2014 which came into operation on ... ... ... ... … … … … 18 April 2014 3 LAWS OF MALAYSIA Act 716 WILDLIFE CONSERVATION ACT 2010 ARRANGEMENT OF SECTIONS PART I PRELIMINARY Section 1. Short title and commencement 2. Application 3. Interpretation PART II APPOINTMENT OF OFFICERS, ETC. 4. Appointment of officers, etc. 5. Delegation of powers 6. Power of Minister to give directions 7. Power of the Director General to issue orders 8. Carrying and use of arms PART III LICENSING PROVISIONS Chapter 1 Requirement for licence, etc. 9. Requirement for licence 4 Laws of Malaysia ACT 716 Section 10. Requirement for permit 11. Requirement for special permit Chapter 2 Application for licence, etc. 12. Application for licence, etc. 13. Additional information or document 14. Grant of licence, etc. 15. Power to impose additional conditions and to vary or revoke conditions 16. Validity of licence, etc. 17. Carrying or displaying licence, etc. 18. Change of particulars 19. Loss of licence, etc. 20. Replacement of licence, etc. 21. Assignment of licence, etc. 22. Return of licence, etc., upon expiry 23. Suspension or revocation of licence, etc. 24. Licence, etc., to be void 25. Appeals Chapter 3 Miscellaneous 26. Hunting by means of shooting 27. No licence during close season 28. Prerequisites to operate zoo, etc. 29. Prohibition of possessing, etc., snares 30. -

Keanekaragaman Reptil Impor Di Yogyakarta
Biota Vol. 1 (3): 117−125, Oktober 2016 ISSN 2527-323X Keanekaragaman Reptil Impor di Yogyakarta Diversity of Imported Reptiles in Yogyakarta Dicky Indar Putranto*, Pramana Yuda, Felicia Zahida Fakultas Teknobiologi, Universitas Atma Jaya Yogyakarta, Jln. Babarsari 44 Yogyakarta 55281 E-mail: [email protected] *Penulis untuk korespondensi Abstract Imported reptiles are in great demand because they have a wide variety of colors. This research is about exotic reptile species in Yogyakarta which are preserved or detached or released in the wild; and their potential impacts for local reptile species in Yogyakarta. This research was conducted in urban sites of Yogyakarta by conducting survey on animal markets, pet shops and reptile owners. This research was conducted from August 1, 2013 to November 30, 2013. Based on the result of the survey of imported reptile data collection in Yogyakarta, there was found 80 species, consisting of one species of pygmy crocodiles (Paleosuchus palpebrosus), 14 species of lizards (Sauria), 21 species of serpentes (Serpentes), and 44 species of turtles (Testudines). Imported reptiles released in nature were found in several numbers of species, namely two Red Eared Sliders (Trachemys scripta elegans), three Chinese Soft-shelled Turtles (Pelodiscus sinensis) and one Corn snake (Pantherophis guttatus). Red Eared Sliders which were released in the wild in such number cannot have a negative impact on local reptiles, but if in large quantities, this species is likely to be a potential competitor for bulus jawa (Amyda cartilaginea) in foraging for food. Chinese Soft-shelled Turtle were released in nature. In such number, it is likely to be a potential competitor for Amyda cartilaginea in foraging for food. -

Membros Da Comissão Julgadora Da Dissertação
UNIVERSIDADE DE SÃO PAULO FACULDADE DE FILOSOFIA, CIÊNCIAS E LETRAS DE RIBEIRÃO PRETO PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA Evolution of the skull shape in extinct and extant turtles Evolução da forma do crânio em tartarugas extintas e viventes Guilherme Hermanson Souza Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo, como parte das exigências para obtenção do título de Mestre em Ciências, obtido no Programa de Pós- Graduação em Biologia Comparada Ribeirão Preto - SP 2021 UNIVERSIDADE DE SÃO PAULO FACULDADE DE FILOSOFIA, CIÊNCIAS E LETRAS DE RIBEIRÃO PRETO PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA COMPARADA Evolution of the skull shape in extinct and extant turtles Evolução da forma do crânio em tartarugas extintas e viventes Guilherme Hermanson Souza Dissertação apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo, como parte das exigências para obtenção do título de Mestre em Ciências, obtido no Programa de Pós- Graduação em Biologia Comparada. Orientador: Prof. Dr. Max Cardoso Langer Ribeirão Preto - SP 2021 Autorizo a reprodução e divulgação total ou parcial deste trabalho, por qualquer meio convencional ou eletrônico, para fins de estudo e pesquisa, desde que citada a fonte. I authorise the reproduction and total or partial disclosure of this work, via any conventional or electronic medium, for aims of study and research, with the condition that the source is cited. FICHA CATALOGRÁFICA Hermanson, Guilherme Evolution of the skull shape in extinct and extant turtles, 2021. 132 páginas. Dissertação de Mestrado, apresentada à Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto/USP – Área de concentração: Biologia Comparada. -

Distribution and Population Status of the Narrow-Headed Softshell Turtle Chitra Spp
The Natural History Journal of Chulalongkorn University 5(1): 31-42, May 2005 ©2005 by Chulalongkorn University Distribution and Population Status of the Narrow-Headed Softshell Turtle Chitra spp. in Thailand ∗ WACHIRA KITIMASAK1,2, , KUMTHORN THIRAKHUPT1, SITDHI BOONYARATPALIN3 AND DON L. MOLL4 1Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10330, THAILAND 2Kanchanaburi Inland Fisheries Research and Development Center, Tha Muang, Kanchanaburi Province 71110, THAILAND 3Department of Fisheries, Kasetklang, Chatuchak, Bangkok 10900, THAILAND 4Department of Biology, Southwest Missouri State University, Springfield, Missouri 65804, USA ABSTRACT.–The distribution range and status of Chitra spp. in Thailand were investigated. Chitra chitra Nutphand, 1986 is so far known only from the Mae Klong and Chao Phraya river systems. Another species, Chitra vandijki McCord and Pritchard, 2002, was reported to occur in the Salween river system located along the Thailand-Myanmar border. At present, the status of Chitra spp. is very rare everywhere and the natural population seems to be declining. Conservation and management action in behalf of this Genus is urgently needed. KEY WORDS: Trionychidae, Chitra chitra, Chitra vandijki, softshell turtle, distribution, conservation Thailand. Information subsequently presented INTRODUCTION by Engstrom et al. (2002), Engstrom and McCord (2002), and McCord and Pritchard The Siamese narrow-headed softshell turtle, (2002) now indicates that populations C. chitra Nutphand, 1986, is probably the representing this species also extend into largest softshell turtle in the world. Pritchard peninsular Malaysia and Indonesia (to Java). Of (2001) estimated the maximum leathery the six major river drainages recognized in carapace length (LCL) of C. chitra as 122 cm. Thailand by Vidthayanon et al. -

Apalone Spinifera Atra (Webb and Legler 1960) – Black Spiny Softshell Turtle, Cuatrociénegas Softshell, Tortuga Concha Blanda, Tortuga Negra De Cuatrociénegas
Conservation Biology of Freshwater Turtles and Tortoises: A Compilation ProjectTrionychidae of the IUCN/SSC — ApaloneTortoise and spinifera Freshwater atra Turtle Specialist Group 021.1 A.G.J. Rhodin, P.C.H. Pritchard, P.P. van Dijk, R.A. Saumure, K.A. Buhlmann, and J.B. Iverson, Eds. Chelonian Research Monographs (ISSN 1088-7105) No. 5, doi:10.3854/crm.5.021.atra.v1.2008 © 2008 by Chelonian Research Foundation • Published 9 August 2008 Apalone spinifera atra (Webb and Legler 1960) – Black Spiny Softshell Turtle, Cuatrociénegas Softshell, Tortuga Concha Blanda, Tortuga Negra de Cuatrociénegas ADRIÁN CERDÁ -ARDUR A 1, FR A N C IS C O SOBERÓN -MOB A R A K 2, SUZ A NNE E. MCGA U G H 3, A ND RI C H A RD C. VO G T 4 1Romero 93 Col. Niños Heroes, C.P. 03440, Mexico D.F. Mexico [[email protected]]; 2Xavier Sorondo 210 Col. Iztaccihuatl, C.P. 03520, Mexico D.F. Mexico [[email protected]]; 3Department of Ecology, Evolution, and Organismal Biology, Iowa State University, Ames, Iowa 50011 USA [[email protected]]; 4CPBA/INPA, Caixa Postal 478, Petropolis, Manaus, Amazonas 69011-970 Brazil [[email protected]] SU mma RY . – Apalone spinifera atra (Family Trionychidae), endemic to the Cuatrociénegas Basin of Coahuila, Mexico, is an enigmatic and severely threatened softshell turtle. On the basis of mor- phology, it has been regarded as a full species (Apalone ater), but by phylogenetic molecular analyses it is currently considered a subspecies of A. spinifera. The discovery of color morphs correlated to substrate coloration in different localities and the recognition of hybridization between A. -

Cantor's Giant Softshell Turtle, Pelochelys Cantorii
M Cantor’s Giant Softshell turtle, Pelochelys cantorii Compiler: Ayushi Jain Suggested citation: Jain, A., Das, A., V. Deepak., Cavada-Blanco, F. 2021. A Survival Blueprint for the Cantor’s Giant Softshell Turtle Pelochelys cantorii in India. EDGE of Existence programme, Zoological Society of London, UK 1. STATUS REVIEW 1.1 Taxonomy: Class : Reptilia Order : Testudines Family : Trionychidae Genus : Pelochelys Species : Pelocheys cantorii (Gray, 1864) Common Name : Cantor’s Giant softshell turtle/ Asian Giant softshell turtle/ Local name : Bheemanama, Paala poovan (Malayalam) Synonyms: Pelochelys clivepalmeri (Hoser, 2014), P. cumingii (Gray, 1864), P. poljakowii (Strauch, 1890), P. telstraorum (Hoser, 2014), P. cantoris (Boulenger, 1889) Pelochelys cantorii (Gray, 1864) is one of the three species in the genus Pelochelys. The other two species are P. bibroni and P. signifera known only from Papua New Guinea and Indonesia (Papua), respectively. P. cantorii has a large distribution across south and south-east Asia (Das, 2008). It is among the largest freshwater turtles in the world with adults reaching a carapace length of around 100 cm (Das, 2008). Sexual dimorphism is present with males having longer and thicker tales than females; something common for other softshell turtles. Females are also larger in size than males (Das, 2008). According to the last IUCN Red List of threatened species assessment for the species, Pelochelys cantorii might hide a complex of several different species (ATTWG, 2000) A B Figure 1. An adult Pelochelys cantorii on the banks of Chandragiri river caught as by-catch in a fishing line (A), and a close-up head shot showing the keratinized sheath or “teeth” of the species (B). -

Nilssonia Leithii (Gray 1872) – Leith's Softshell Turtle
Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project ofTrionychidae the IUCN/SSC Tortoise— Nilssonia and Freshwater leithii Turtle Specialist Group 075.1 A.G.J. Rhodin, P.C.H. Pritchard, P.P. van Dijk, R.A. Saumure, K.A. Buhlmann, J.B. Iverson, and R.A. Mittermeier, Eds. Chelonian Research Monographs (ISSN 1088-7105) No. 5, doi:10.3854/crm.5.075.leithii.v1.2014 © 2014 by Chelonian Research Foundation • Published 17 February 2014 Nilssonia leithii (Gray 1872) – Leith’s Softshell Turtle INDRANE I L DAS 1, SHASHWAT SI RS I 2, KARTH ik EYAN VASUDE V AN 3, AND B.H.C.K. MURTHY 4 1Institute of Biodiversity and Environmental Conservation, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia [[email protected]]; 2Turtle Survival Alliance-India, D-1/316 Sector F, Janakipuram, Lucknow 226 021, India [[email protected]]; 3Laboratory for Conservation of Endangered Species, Centre for Cellular and Molecular Biology, Pillar 162, PVNR Expressway, Hyderguda, Hyderabad 500 048, India [[email protected]]; 4Zoological Survey of India, J.L. Nehru Road, Kolkata 700 016, India [[email protected]] SU mm ARY . – Leith’s Softshell Turtle, Nilssonia leithii (Family Trionychidae), is a large turtle, known to attain at least 720 mm in carapace length (bony disk plus fibrocartilage flap), and possibly as much as 1000 mm. The species inhabits the rivers and reservoirs of southern peninsular India, replacing the more familiar Indian Softshell Turtle, N. gangetica, of northern India. The turtle is apparently rare within its range, even within protected areas, which is suspected to be due to a past history of exploitation. -

Identification of Sex Using SBNO1 Gene
Journal of Genetics (2019) 98:36 © Indian Academy of Sciences https://doi.org/10.1007/s12041-018-1048-z RESEARCH NOTE Identification of sex using SBNO1 gene in the Chinese softshell turtle, Pelodiscus sinensis (Trionychidae) LAN ZHAO, XIN WANG, QIU-HONG WAN and SHENG-GUO FANG∗ The Key Laboratory of Conservation Biology for Endangered Wildlife of the Ministry of Education and State Conservation Centre for Gene Resources of Endangered Wildlife, College of Life Sciences, Zhejiang University, Hangzhou 310058, People’s Republic of China *For correspondence. E-mail: [email protected]. Received 20 June 2018; revised 17 September 2018; accepted 19 September 2018; published online 11 April 2019 Abstract. The Chinese softshell turtle exhibits ZZ/ZW sex determination. To identify the sex of embryos, juvenile and adult individuals, we designed two pairs of polymerase chain reaction primers, SB1-196, which amplifies a fragment of 196 bp in the female and the other, CK1-482, which amplifies the 482-bp fragment in both the sexes. It is validated in 24 adult turtles of known sex, sampled from three different locations. This one-step sexing technique is rapid and easy to perform and is reported for the first time. Keywords. polymerase chain reaction; sex identification; sex chromosome; molecular sexing; reptile; Chinese softshell turtle. Introduction rapid method for identifying the sex of this species will contribute to development of breeding and conservation The Chinese softshell turtle, Pelodiscus sinensis (family programmes. Trionychidae, suborder Cryptodira), possesses heteromor- In the present study, a pair of primers is designed phic sex chromosomes (ZZ male, ZW female) (Kawai et al. -
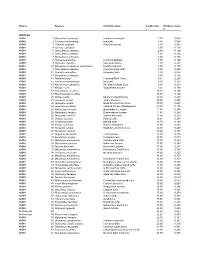
PDF File Containing Table of Lengths and Thicknesses of Turtle Shells And
Source Species Common name length (cm) thickness (cm) L t TURTLES AMNH 1 Sternotherus odoratus common musk turtle 2.30 0.089 AMNH 2 Clemmys muhlenbergi bug turtle 3.80 0.069 AMNH 3 Chersina angulata Angulate tortoise 3.90 0.050 AMNH 4 Testudo carbonera 6.97 0.130 AMNH 5 Sternotherus oderatus 6.99 0.160 AMNH 6 Sternotherus oderatus 7.00 0.165 AMNH 7 Sternotherus oderatus 7.00 0.165 AMNH 8 Homopus areolatus Common padloper 7.95 0.100 AMNH 9 Homopus signatus Speckled tortoise 7.98 0.231 AMNH 10 Kinosternon subrabum steinochneri Florida mud turtle 8.90 0.178 AMNH 11 Sternotherus oderatus Common musk turtle 8.98 0.290 AMNH 12 Chelydra serpentina Snapping turtle 8.98 0.076 AMNH 13 Sternotherus oderatus 9.00 0.168 AMNH 14 Hardella thurgi Crowned River Turtle 9.04 0.263 AMNH 15 Clemmys muhlenbergii Bog turtle 9.09 0.231 AMNH 16 Kinosternon subrubrum The Eastern Mud Turtle 9.10 0.253 AMNH 17 Kinixys crosa hinged-back tortoise 9.34 0.160 AMNH 18 Peamobates oculifers 10.17 0.140 AMNH 19 Peammobates oculifera 10.27 0.140 AMNH 20 Kinixys spekii Speke's hinged tortoise 10.30 0.201 AMNH 21 Terrapene ornata ornate box turtle 10.30 0.406 AMNH 22 Terrapene ornata North American box turtle 10.76 0.257 AMNH 23 Geochelone radiata radiated tortoise (Madagascar) 10.80 0.155 AMNH 24 Malaclemys terrapin diamondback terrapin 11.40 0.295 AMNH 25 Malaclemys terrapin Diamondback terrapin 11.58 0.264 AMNH 26 Terrapene carolina eastern box turtle 11.80 0.259 AMNH 27 Chrysemys picta Painted turtle 12.21 0.267 AMNH 28 Chrysemys picta painted turtle 12.70 0.168 AMNH 29 -

Trace Element Concentrations in European Pond Turtles (Emys Orbicularis) from Brenne Natural Park, France
Author's personal copy Bulletin of Environmental Contamination and Toxicology (2018) 101:300–304 https://doi.org/10.1007/s00128-018-2376-7 Trace Element Concentrations in European Pond Turtles (Emys orbicularis) from Brenne Natural Park, France Héloïse Guillot1 · Xavier Bonnet1 · Paco Bustamante2 · Carine Churlaud2 · Jacques Trotignon3 · François Brischoux1 Received: 25 May 2018 / Accepted: 2 June 2018 / Published online: 8 June 2018 © Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract We assessed trace elements concentration in European pond turtle (Emys orbicularis) from Brenne Natural Park (France). We sampled road-killed turtles (N = 46) to measure the concentrations of 4 non-essential (Ag, Cd, Hg, and Pb) and 10 essential (As, Co, Cr, Cu, Fe, Mn, Ni, Se, V, and Zn) elements in muscle, skin, liver and claws. Body size or sex did not influence the concentrations of most elements; except for Hg (liver, skin and claws) and Zn (muscle) which increased with body size. We found relatively high concentrations of Hg and Zn, possibly linked to fish farming. This result deserves future investigations to evaluate possible ecotoxicological effects onE. orbicularis. Keywords Trace elements · Contamination · Wetlands · Emys orbicularis Wetlands are important habitats for biodiversity, yet they to aquatic ecosystems because of their high toxicity, persis- are among the most endangered ecosystems in the world, tence, bioaccumulation in organisms and biomagnification suffering from a drastic reduction of their surface and from across trophic levels (Agarwal 2009). a degradation of water quality (Schneider et al. 2017). Freshwater turtles are suitable organisms to survey con- Monitoring environmental contaminations in wetlands is tamination levels in complex aquatic ecosystems (Overmann difficult because they are connected to complex and large and Krajicek 1995; Ayub et al. -

REPRODUCCIÓN DE Trachemys Callirostris Callirostris (EMYDIDAE) EN AMBIENTES GENERADOS POR LA MINERÍA EN LA GUAJIRA, COLOMBIA
SEDE BOGOTÁ ACTA BIOLÓGICA COLOMBIANA FACULTAD DE CIENCIAS DEPARTAMENTO DE BIOLOGÍA ARTÍCULO DE INVESTIGACIÓN REPRODUCCIÓN DE Trachemys callirostris callirostris (EMYDIDAE) EN AMBIENTES GENERADOS POR LA MINERÍA EN LA GUAJIRA, COLOMBIA Reproduction of Trachemys callirostris callirostris (Emydidae) in environments created by mining in La Guajira, Colombia CINDY LEGUÍZAMO-PARDO1, M. Sc.; MARÍA ARGENIS BONILLA GÓMEZ2, Ph. D. 1 Grupo Biología de Organismos Tropicales, Universidad Nacional de Colombia, Bogotá, Colombia. [email protected] 2 Profesora Asociada, Departamento de Biología, Universidad Nacional de Colombia, Grupo Biología de Organismos Tropicales (BIOTUN), Bogotá, Colombia. [email protected] Autor de correspondencia: Cindy Leguízamo Pardo, [email protected] Recibido 16 de enero de 2014, aceptado con modificaciones 22 de marzo de 2014, fecha de reenvío 19 de abril de 2014. Citation / Citar este artículo como: LEGUÍZAMO-PARDO C, BONILLA GÓMEZ MA. Reproducción de Trachemys callirostris callirostris (Emydidade) en ambientes generados por la minería en La Guajira, Colombia. Acta biol. Colomb. 2014;19(3):363-380. RESUMEN La tortuga hicotea (Trachemys callirostris callirostris) es una subespecie sometida a una alta extracción en Colombia, de la cual no se conoce nada sobre su reproducción en zonas altamente alteradas con bajo impacto por la cacería. Para ello, en tres ambientes acuáticos generados por la minería de carbón en la mina del Cerrejón, departamento de La Guajira, estudiamos algunas características reproductivas de la hicotea durante el periodo reproductivo de 2011 (marzo a junio). Solamente en las lagunas de estabilización registramos un éxito de eclosión positivo (56,9 %). En el embalse de minería, la tasa de depredación de 100 % fue el factor limitante del éxito de eclosión, por lo que recomendamos el aislamiento de los nidos del principal depredador (zorro patón: Procyon cancrivorus) y el traslado de nidadas para su incubación ex-situ. -

Herpetofaunal Communities in Ephemeral Wetlands Embedded Within Longleaf Pine Flatwoods of the Gulf Coastal Plain
20162016 SOUTHEASTERNSoutheastern NaturalistNATURALIST 15(3):431–447Vol. 15, No. 3 K.J. Erwin, H.C. Chandler, J.G. Palis, T.A. Gorman, and C.A. Haas Herpetofaunal Communities in Ephemeral Wetlands Embedded within Longleaf Pine Flatwoods of the Gulf Coastal Plain Kenneth J. Erwin1, Houston C. Chandler1,2,*, John G. Palis3, Thomas A. Gorman1,4, and Carola A. Haas1 Abstract - Ephemeral wetlands surrounded by Pinus palustris (Longleaf Pine) flatwoods support diverse herpetofaunal communities and provide important breeding habitat for many species. We sampled herpetofauna in 3 pine flatwoods wetlands on Eglin Air Force Base, Okaloosa County, FL, over 2 time periods (1 wetland [1] from 1993 to1995 and 2 wetlands [2 and 3] from 2010 to 2015) using drift fences that completely encircled each wetland. We documented 37, 46, and 43 species of amphibians and reptiles at wetlands 1, 2, and 3, respectively. Herpetofaunal communities were remarkably similar across all 3 wetlands (Sorenson Index values > 0.97) despite sampling that occurred 15–20 years apart on wetlands located approximately 10 km apart. Ambystoma bishopi (Reticulated Flat- woods Salamander), Pseudacris ornata (Ornate Chorus Frog), and Eurycea quadridigitata (Dwarf Salamander), all species of conservation concern, were captured at all 3 wetlands, indicating that these wetlands provide habitat for specialist species. Overall, habitat con- servation and management has succeeded in maintaining suitable habitat for herpetofauna in recently surveyed wetlands, despite continued range-wide threats from changes to his- toric fire regimes and climate change. Introduction Ephemeral wetlands often support diverse amphibian and reptile communities (Dodd and Cade 1998, Gibbons et al. 2006, Means et al.