1) Introduction 2) Ionization Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Physical Mechanism of Superconductivity
Physical Mechanism of Superconductivity Part 1 – High Tc Superconductors Xue-Shu Zhao, Yu-Ru Ge, Xin Zhao, Hong Zhao ABSTRACT The physical mechanism of superconductivity is proposed on the basis of carrier-induced dynamic strain effect. By this new model, superconducting state consists of the dynamic bound state of superconducting electrons, which is formed by the high-energy nonbonding electrons through dynamic interaction with their surrounding lattice to trap themselves into the three - dimensional potential wells lying in energy at above the Fermi level of the material. The binding energy of superconducting electrons dominates the superconducting transition temperature in the corresponding material. Under an electric field, superconducting electrons move coherently with lattice distortion wave and periodically exchange their excitation energy with chain lattice, that is, the superconducting electrons transfer periodically between their dynamic bound state and conducting state, so the superconducting electrons cannot be scattered by the chain lattice, and supercurrent persists in time. Thus, the intrinsic feature of superconductivity is to generate an oscillating current under a dc voltage. The wave length of an oscillating current equals the coherence length of superconducting electrons. The coherence lengths in cuprates must have the value equal to an even number times the lattice constant. A superconducting material must simultaneously satisfy the following three criteria required by superconductivity. First, the superconducting materials must possess high – energy nonbonding electrons with the certain concentrations required by their coherence lengths. Second, there must exist three – dimensional potential wells lying in energy at above the Fermi level of the material. Finally, the band structure of a superconducting material should have a widely dispersive antibonding band, which crosses the Fermi level and runs over the height of the potential wells to ensure the normal state of the material being metallic. -

Contents • Introduction to Proteomics and Mass Spectrometry
Contents • Introduction to Proteomics and Mass spectrometry - What we need to know in our quest to explain life - Fundamental things you need to know about mass spectrometry • Interfaces and ion sources - Electrospray ionization (ESI) - conventional and nanospray - Heated nebulizer atmospheric pressure chemical ionization - Matrix assisted laser desorption • Types of MS analyzers - Magnetic sector - Quadrupole - Time-of-flight - Hybrid - Ion trap In the next step in our quest to explain what is life The human genome project has largely been completed and many other genomes are surrendering to the gene sequencers. However, all this knowledge does not give us the information that is needed to explain how living cells work. To do that, we need to study proteins. In 2002, mass spectrometry has developed to the point where it has the capacity to obtain the "exact" molecular weight of many macromolecules. At the present time, this includes proteins up to 150,000 Da. Proteins of higher molecular weights (up to 500,000 Da) can also be studied by mass spectrometry, but with less accuracy. The paradigm for sequencing of peptides and identification of proteins has changed – because of the availability of the human genome database, peptides can be identified merely by their masses or by partial sequence information, often in minutes, not hours. This new capacity is shifting the emphasis of biomedical research back to the functional aspects of cell biochemistry, the expression of particular sets of genes and their gene products, the proteins of the cell. These are the new goals of the biological scientist: o to know which proteins are expressed in each cell, preferably one cell at a time o to know how these proteins are modified, information that cannot necessarily be deduced from the nucleotide sequence of individual genes. -

Electron Ionization
Chapter 6 Chapter 6 Electron Ionization I. Introduction ......................................................................................................317 II. Ionization Process............................................................................................317 III. Strategy for Data Interpretation......................................................................321 1. Assumptions 2. The Ionization Process IV. Types of Fragmentation Pathways.................................................................328 1. Sigma-Bond Cleavage 2. Homolytic or Radical-Site-Driven Cleavage 3. Heterolytic or Charge-Site-Driven Cleavage 4. Rearrangements A. Hydrogen-Shift Rearrangements B. Hydride-Shift Rearrangements V. Representative Fragmentations (Spectra) of Classes of Compounds.......... 344 1. Hydrocarbons A. Saturated Hydrocarbons 1) Straight-Chain Hydrocarbons 2) Branched Hydrocarbons 3) Cyclic Hydrocarbons B. Unsaturated C. Aromatic 2. Alkyl Halides 3. Oxygen-Containing Compounds A. Aliphatic Alcohols B. Aliphatic Ethers C. Aromatic Alcohols D. Cyclic Ethers E. Ketones and Aldehydes F. Aliphatic Acids and Esters G. Aromatic Acids and Esters 4. Nitrogen-Containing Compounds A. Aliphatic Amines B. Aromatic Compounds Containing Atoms of Nitrogen C. Heterocyclic Nitrogen-Containing Compounds D. Nitro Compounds E. Concluding Remarks on the Mass Spectra of Nitrogen-Containing Compounds 5. Multiple Heteroatoms or Heteroatoms and a Double Bond 6. Trimethylsilyl Derivative 7. Determining the Location of Double Bonds VI. Library -

Modern Mass Spectrometry
Modern Mass Spectrometry MacMillan Group Meeting 2005 Sandra Lee Key References: E. Uggerud, S. Petrie, D. K. Bohme, F. Turecek, D. Schröder, H. Schwarz, D. Plattner, T. Wyttenbach, M. T. Bowers, P. B. Armentrout, S. A. Truger, T. Junker, G. Suizdak, Mark Brönstrup. Topics in Current Chemistry: Modern Mass Spectroscopy, pp. 1-302, 225. Springer-Verlag, Berlin, 2003. Current Topics in Organic Chemistry 2003, 15, 1503-1624 1 The Basics of Mass Spectroscopy ! Purpose Mass spectrometers use the difference in mass-to-charge ratio (m/z) of ionized atoms or molecules to separate them. Therefore, mass spectroscopy allows quantitation of atoms or molecules and provides structural information by the identification of distinctive fragmentation patterns. The general operation of a mass spectrometer is: "1. " create gas-phase ions "2. " separate the ions in space or time based on their mass-to-charge ratio "3. " measure the quantity of ions of each mass-to-charge ratio Ionization sources ! Instrumentation Chemical Ionisation (CI) Atmospheric Pressure CI!(APCI) Electron Impact!(EI) Electrospray Ionization!(ESI) SORTING DETECTION IONIZATION OF IONS OF IONS Fast Atom Bombardment (FAB) Field Desorption/Field Ionisation (FD/FI) Matrix Assisted Laser Desorption gaseous mass ion Ionisation!(MALDI) ion source analyzer transducer Thermospray Ionisation (TI) Analyzers quadrupoles vacuum signal Time-of-Flight (TOF) pump processor magnetic sectors 10-5– 10-8 torr Fourier transform and quadrupole ion traps inlet Detectors mass electron multiplier spectrum Faraday cup Ionization Sources: Classical Methods ! Electron Impact Ionization A beam of electrons passes through a gas-phase sample and collides with neutral analyte molcules (M) to produce a positively charged ion or a fragment ion. -
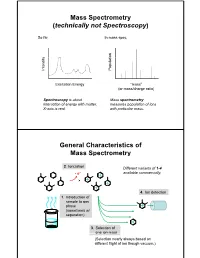
Mass Spectrometry (Technically Not Spectroscopy)
Mass Spectrometry (technically not Spectroscopy) So far, In mass spec, on y Populati Intensit Excitation Energy “mass” (or mass/charge ratio) Spectroscopy is about Mass spectrometry interaction of energy with matter. measures population of ions X-axis is real. with particular mass. General Characteristics of Mass Spectrometry 2. Ionization Different variants of 1-4 -e- available commercially. 4. Ion detection 1. Introduction of sample to gas phase (sometimes w/ separation) 3. Selection of one ion mass (Selection nearly always based on different flight of ion though vacuum.) General Components of a Mass Spectrometer Lots of choices, which can be mixed and matched. direct injection The Mass Spectrum fragment “daughter” ions M+ “parent” mass Sample Introduction: Direc t Inser tion Prob e If sample is a liquid, sample can also be injected directly into ionization region. If sample isn’t pure, get multiple parents (that can’t be distinguished from fragments). Capillary Column Introduction Continous source of molecules to spectrometer. detector column (including GC, LC, chiral, size exclusion) • Signal intensity depends on both amount of molecule and ionization efficiency • To use quantitatively, must calibrate peaks with respect eltilution time ttlitotal ion curren t to quantity eluted (TIC) over time Capillary Column Introduction Easy to interface with gas or liquid chromatography. TIC trace elution time time averaged time averaged mass spectrum mass spectrum Methods of Ionization: Electron Ionization (EI) 1 - + - 1 M + e (kV energy) M + 2e Fragmentation in Electron Ionization daughter ion (observed in spectrum) neutral fragment (not observed) excited parent at electron at electron energy of energy of 15 eV 70 e V Lower electron energy yields less fragmentation, but also less signal. -

An Introduction to Mass Spectrometry
An Introduction to Mass Spectrometry by Scott E. Van Bramer Widener University Department of Chemistry One University Place Chester, PA 19013 [email protected] http://science.widener.edu/~svanbram revised: September 2, 1998 © Copyright 1997 TABLE OF CONTENTS INTRODUCTION ........................................................... 4 SAMPLE INTRODUCTION ....................................................5 Direct Vapor Inlet .......................................................5 Gas Chromatography.....................................................5 Liquid Chromatography...................................................6 Direct Insertion Probe ....................................................6 Direct Ionization of Sample ................................................6 IONIZATION TECHNIQUES...................................................6 Electron Ionization .......................................................7 Chemical Ionization ..................................................... 9 Fast Atom Bombardment and Secondary Ion Mass Spectrometry .................10 Atmospheric Pressure Ionization and Electrospray Ionization ....................11 Matrix Assisted Laser Desorption/Ionization ................................ 13 Other Ionization Methods ................................................13 Self-Test #1 ...........................................................14 MASS ANALYZERS .........................................................14 Quadrupole ............................................................15 -

Ion-To-Neutral Ratios and Thermal Proton Transfer in Matrix-Assisted Laser Desorption/Ionization
B American Society for Mass Spectrometry, 2015 J. Am. Soc. Mass Spectrom. (2015) 26:1242Y1251 DOI: 10.1007/s13361-015-1112-3 RESEARCH ARTICLE Ion-to-Neutral Ratios and Thermal Proton Transfer in Matrix-Assisted Laser Desorption/Ionization I-Chung Lu,1 Kuan Yu Chu,1,2 Chih-Yuan Lin,1 Shang-Yun Wu,1 Yuri A. Dyakov,1 Jien-Lian Chen,1 Angus Gray-Weale,3 Yuan-Tseh Lee,1,2 Chi-Kung Ni1,4 1Institute of Atomic and Molecular Sciences, Academia Sinica, Taipei, 10617, Taiwan 2Department of Chemistry, National Taiwan University, Taipei, 10617, Taiwan 3School of Chemistry, University of Melbourne, Melbourne, VIC 3010, Australia 4Department of Chemistry, National Tsing Hua University, Hsinchu, 30013, Taiwan Abstract. The ion-to-neutral ratios of four commonly used solid matrices, α-cyano-4- hydroxycinnamic acid (CHCA), 2,5-dihydroxybenzoic acid (2,5-DHB), sinapinic acid (SA), and ferulic acid (FA) in matrix-assisted laser desorption/ionization (MALDI) at 355 nm are reported. Ions are measured using a time-of-flight mass spectrometer combined with a time-sliced ion imaging detector. Neutrals are measured using a rotatable quadrupole mass spectrometer. The ion-to-neutral ratios of CHCA are three orders of magnitude larger than those of the other matrices at the same laser fluence. The ion-to-neutral ratios predicted using the thermal proton transfer model are similar to the experimental measurements, indicating that thermal proton transfer reactions play a major role in generating ions in ultraviolet-MALDI. Keywords: MALDI, Ionization mechanism, Thermal proton transfer, Ion-to-neutral ratio Received: 25 August 2014/Revised: 15 February 2015/Accepted: 16 February 2015/Published Online: 8 April 2015 Introduction in solid state UV-MALDI. -

An Organic Chemist's Guide to Electrospray Mass Spectrometric
molecules Review An Organic Chemist’s Guide to Electrospray Mass Spectrometric Structure Elucidation Arnold Steckel 1 and Gitta Schlosser 2,* 1 Hevesy György PhD School of Chemistry, ELTE Eötvös Loránd University, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary; [email protected] 2 Department of Analytical Chemistry, ELTE Eötvös Loránd University, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary * Correspondence: [email protected] Received: 16 January 2019; Accepted: 8 February 2019; Published: 10 February 2019 Abstract: Tandem mass spectrometry is an important tool for structure elucidation of natural and synthetic organic products. Fragmentation of odd electron ions (OE+) generated by electron ionization (EI) was extensively studied in the last few decades, however there are only a few systematic reviews available concerning the fragmentation of even-electron ions (EE+/EE−) produced by the currently most common ionization techniques, electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). This review summarizes the most important features of tandem mass spectra generated by collision-induced dissociation fragmentation and presents didactic examples for the unexperienced users. Keywords: tandem mass spectrometry; MS/MS fragmentation; collision-induced dissociation; CID; ESI; structure elucidation 1. Introduction Electron ionization (EI), a hard ionization technique, is the method of choice for analyses of small (<1000 Da), nonpolar, volatile compounds. As its name implies, the technique involves ionization by electrons with ~70 eV energy. This energy is high enough to yield very reproducible mass spectra with a large number of fragments. However, these spectra frequently lack the radical type molecular ions (M+) due to the high internal energy transferred to the precursors [1]. -

Itwg Guideline Thermal Ionisation Mass Spectrometry (Tims) Executive Summary
NUCLEAR FORENSICS INTERNATIONAL TECHNICAL WORKING GROUP ITWG GUIDELINE THERMAL IONISATION MASS SPECTROMETRY (TIMS) EXECUTIVE SUMMARY Thermal Ionisation Mass Spectrometry (TIMS) is used for isotopic composition measurement of elements having relatively low ionisation potentials (e.g. Sr, Pb, actinides and rare earth elements). Also the concentration of an element can be determined by TIMS using the isotope dilution technique by adding to the sample a known amount of a “spike” [1]. TIMS is a single element analysis technique, thus it is recommended to separate all other elements from the sample before the measurement as they may cause mass interferences or affect the ionisation behaviour of the element of interest [2]. This document was designed and printed at Lawrence Livermore National Laboratory in 2017 with the permission of the Nuclear Forensics International Technical Working Group (ITWG). ITWG Guidelines are intended as consensus-driven best-practices documents. These documents are general rather than prescriptive, and they are not intended to replace any specific laboratory operating procedures. 1. INTRODUCTION The filament configuration in the ion source can be either single, double, or triple filament. In the single In TIMS, a liquid sample (typically in the diluted nitric acid filament configuration, the same filament serves both media) is deposited on a metal ribbon (called a filament for evaporation and ionisation. In the double and triple made of rhenium, tantalum, or tungsten) and dried. filament configurations, the evaporation of the sample The filament is then heated in the vacuum of the mass and the ionisation take place in separate filaments (Fig. spectrometer, causing atoms in the sample to evaporate 2). -

Methods of Ion Generation
Chem. Rev. 2001, 101, 361−375 361 Methods of Ion Generation Marvin L. Vestal PE Biosystems, Framingham, Massachusetts 01701 Received May 24, 2000 Contents I. Introduction 361 II. Atomic Ions 362 A. Thermal Ionization 362 B. Spark Source 362 C. Plasma Sources 362 D. Glow Discharge 362 E. Inductively Coupled Plasma (ICP) 363 III. Molecular Ions from Volatile Samples. 364 A. Electron Ionization (EI) 364 B. Chemical Ionization (CI) 365 C. Photoionization (PI) 367 D. Field Ionization (FI) 367 IV. Molecular Ions from Nonvolatile Samples 367 Marvin L. Vestal received his B.S. and M.S. degrees, 1958 and 1960, A. Spray Techniques 367 respectively, in Engineering Sciences from Purdue Univesity, Layfayette, IN. In 1975 he received his Ph.D. degree in Chemical Physics from the B. Electrospray 367 University of Utah, Salt Lake City. From 1958 to 1960 he was a Scientist C. Desorption from Surfaces 369 at Johnston Laboratories, Inc., in Layfayette, IN. From 1960 to 1967 he D. High-Energy Particle Impact 369 became Senior Scientist at Johnston Laboratories, Inc., in Baltimore, MD. E. Low-Energy Particle Impact 370 From 1960 to 1962 he was a Graduate Student in the Department of Physics at John Hopkins University. From 1967 to 1970 he was Vice F. Low-Energy Impact with Liquid Surfaces 371 President at Scientific Research Instruments, Corp. in Baltimore, MD. From G. Flow FAB 371 1970 to 1975 he was a Graduate Student and Research Instructor at the H. Laser Ionization−MALDI 371 University of Utah, Salt Lake City. From 1976 to 1981 he became I. -

How the Saha Ionization Equation Was Discovered
How the Saha Ionization Equation Was Discovered Arnab Rai Choudhuri Department of Physics, Indian Institute of Science, Bangalore – 560012 Introduction Most youngsters aspiring for a career in physics research would be learning the basic research tools under the guidance of a supervisor at the age of 26. It was at this tender age of 26 that Meghnad Saha, who was working at Calcutta University far away from the world’s major centres of physics research and who never had a formal training from any research supervisor, formulated the celebrated Saha ionization equation and revolutionized astrophysics by applying it to solve some long-standing astrophysical problems. The Saha ionization equation is a standard topic in statistical mechanics and is covered in many well-known textbooks of thermodynamics and statistical mechanics [1–3]. Professional physicists are expected to be familiar with it and to know how it can be derived from the fundamental principles of statistical mechanics. But most professional physicists probably would not know the exact nature of Saha’s contributions in the field. Was he the first person who derived and arrived at this equation? It may come as a surprise to many to know that Saha did not derive the equation named after him! He was not even the first person to write down this equation! The equation now called the Saha ionization equation appeared in at least two papers (by J. Eggert [4] and by F.A. Lindemann [5]) published before the first paper by Saha on this subject. The story of how the theory of thermal ionization came into being is full of many dramatic twists and turns. -

C7895 Mass Spectrometry of Biomolecules Schedule of Lectures
C7895 Mass Spectrometry of Biomolecules Schedule of lectures For schedule, please see a separate file with the course outline. Jan Preisler Consulting The last lecture. Please contact me in advance to make an appointment. Chemistry Dept. 312A14, tel.: 54949 6629, [email protected] This material is just an outline; students are advised to print this outline and write down notes durin the lectures. The material will be updated during The course is focused on mass spectrometry of biomolecules, i.e. ionization the semester. techniques MALDI and ESI, modern mass analyzers, such as time-of-flight MS or ion traps and bioanalytical applications. However, the course covers much broader area, including inorganic ionization techniques, virtually all Supporting study material: types of mass analyzers and hardware in mass spectrometry. • J. Gross, Mass Spectrometry, 3rd ed. Springer-Verlag, 2017 • J. Greaves, J. Roboz: Mass Spectrometry for the Novice, CRC Press, 2013 • Edmond de Hoffmann, Vincent Stroobant: Mass Spectrometry: Principles and Applications, 3rd Edition, John Wiley & Sons, 2007 Mass spectrometry of biomolecules 2018 1 Mass spectrometry of biomolecules 2018 2 1 2 Content I. Introduction 1 II. Ionization methods and sample introduction III. Mass analyzers IV. Biological applications of MS V. Example problems Introduction to Mass spectrometry. Brief History of MS. A Survey of Methods and Instrumentation. Basic Concepts in MS: Resolution, Sensitivity. Isotope patterns of organic molecules. Ionization Techniques and Sample Introductin. Electron Impact Mass spectrometry of biomolecules 2018 3 Ionization (EI). Chemical Ionization (CI) 3 4 I. Introduction Study Material • Information sources about mass spectrometry Lecture notes • Brief history of mass spectrometry, a survey of methods and Advice: please take notes, but do not copy the slides; the English slides will instrumentation be provided at the end of the semester.