Study of Intermolecular Interaction in Binary Mixtures of Cumene with Benzene, Toluene and Acetone at 308K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Improved Process for the Production of Cumene
Europaisches Patentamt European Patent Office © Publication number: 0 537 389 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 91309531.1 int. Ci.5; C07C 2/66, C07C 2/86, C07C 6/12, C07C 15/085 (§) Date of filing: 16.10.91 ® Date of publication of application: @ Applicant: Council of Scientific and Industrial 21.04.93 Bulletin 93/16 Research Rafi Marg @ Designated Contracting States: New Delhi 110 001 (IN) BE DE FR GB IT NL @ Inventor: Pradhan, Ajit Ramchandra National Chemical Laboratory Pune-411008, Maharashtra(IN) Inventor: Rao, Bollapragad Seshagiri National Chemical Laboratory Pune-411008, Maharashtra(IN) 0 Representative: Collier, Jeremy Austin Grey et al J.A.Kemp & Co., 14 South Square, Gray's Inn London WC1R 5LX (GB) 0 An improved process for the production of cumene. © An improved process is disclosed for the preparation of cumene. Cumene is prepared by reacting benzene with a propylating agent in the presence of a catalyst containing metal loaded Zeolite EU-1 in a reactor in the range of a temperature of 150 to 250 °C and a pressure of 1 to 35 atmospheres, the propyl and diisopropylben- zene so formed are separated from the reactor effluent by conventional methods. The diisopropylbenzene is recycled back to the reactor. Simultaneous alkylation and transalkylation reactions occur in a single catalyst bed containing Zeolite EU-1 with a feed containing benzene, propylene and diisopropylbenzene. Cumenes are important chemical precursors in the production of detergents and polymers among others. Oi CO CO IV CO m Rank Xerox (UK) Business Services (3. 10/3.5x/3.0. -

ECO-Ssls for Pahs
Ecological Soil Screening Levels for Polycyclic Aromatic Hydrocarbons (PAHs) Interim Final OSWER Directive 9285.7-78 U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 June 2007 This page intentionally left blank TABLE OF CONTENTS 1.0 INTRODUCTION .......................................................1 2.0 SUMMARY OF ECO-SSLs FOR PAHs......................................1 3.0 ECO-SSL FOR TERRESTRIAL PLANTS....................................4 5.0 ECO-SSL FOR AVIAN WILDLIFE.........................................8 6.0 ECO-SSL FOR MAMMALIAN WILDLIFE..................................8 6.1 Mammalian TRV ...................................................8 6.2 Estimation of Dose and Calculation of the Eco-SSL ........................9 7.0 REFERENCES .........................................................16 7.1 General PAH References ............................................16 7.2 References Used for Derivation of Plant and Soil Invertebrate Eco-SSLs ......17 7.3 References Rejected for Use in Derivation of Plant and Soil Invertebrate Eco-SSLs ...............................................................18 7.4 References Used in Derivation of Wildlife TRVs .........................25 7.5 References Rejected for Use in Derivation of Wildlife TRV ................28 i LIST OF TABLES Table 2.1 PAH Eco-SSLs (mg/kg dry weight in soil) ..............................4 Table 3.1 Plant Toxicity Data - PAHs ..........................................5 Table 4.1 -

Specifications Guide Americas Petrochemicals Latest Update: July 2020
Specifications Guide Americas Petrochemicals Latest update: July 2020 Definitions of the trading locations for which Platts publishes daily indexes or assessments 2 Olefins 3 US aromatics 6 Latin American aromatics 8 US polymers 10 Latin American polymers 13 US intermediates 16 US hydrocarbon solvents 17 US chlor alkali 18 US oxygenated solvents 19 Liquid and gas chemical freight 21 Global petrochemical indices 22 Revision history 23 www.spglobal.com/platts Specifications Guide Americas Petrochemicals: July 2020 DEFINITIONS OF THE TRADING LOCATIONS FOR WHICH PLATTS PUBLISHES DAILY INDEXES OR ASSESSMENTS The following specifications guide contains the primary specifications for S&P Global Platts petrochemical assessments in the Americas. All the assessments listed here employ Platts Assessments Methodology, as published at https://www.spglobal.com/platts/plattscontent/_assets/_files/en/our-methodology/methodology-specifications/platts-assessments-methodology-guide.pdf. These guides are designed to give Platts subscribers as much information as possible about a wide range of methodology and specification questions. This guide is current at the time of publication. Platts may issue further updates and enhancements to this methodology and will announce these to subscribers through its usual publications of record. Such updates will be included in the next version of this guide. Platts editorial staff and managers are available to provide guidance when assessment issues require clarification. OLEFINS Assessment CURRENCY CODE Mavg Wavg TYPE -

CUMENE Cumene Is the Main Feedstock for Phenol/Acetone
CUMENE Cumene is the main feedstock for phenol/acetone production and demand is driven by market growth for the derivatives of phenol and acetone such as bis-phenol A, phenolic resins and caprolactam. Other uses for cumene are as a thinner for paints, lacquers and enamels, and as a constituent of some petroleum-based solvents. It is also used in manufacturing polymerisation catalysts, catalyst for acrylic and polyester type resins, and as a raw material for peroxides and oxidation catalysts. Cumene is considered a primary skin and eye irritant and excessive exposure can lead to headaches and narcosis. Cumene is stable at room temperature but must be kept well away from oxidising agents. It is a fire and explosion hazard and above 31oC explosive vapour/air mixtures may be formed. Cumene is released to the environment as a result of its production and processing, from petroleum refining and the evaporation and combustion of petroleum products and by the use of a variety of products containing cumene. When released to the atmosphere, vapour phase cumene will react with photochemically generated hydroxyl radicals with an estimated half-life of 25 hours in polluted atmospheres and 49 hours in normal atmospheres. Cumene is a contaminant of air, sediments and surface, drinking and groundwater and a natural constituent of a variety of foods and vegetation. ICIS pricing quotes cumene in the USA. Frequency: Published weekly on Fridays. Cumene (USA) Weekly Price Assessments: Cumene Contract Prices FOB monthly (US CTS/LB & conversion to USD/MT): General information: Assessment window: Assessments are based on information supplied by market participants through the week up to 1800 hours in Houston on Fridays. -

Toxicological Profile for Ethylbenzene
ETHYLBENZENE 151 5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL 5.1 PRODUCTION Ethylbenzene is primarily produced by the alkylation of benzene with ethylene in liquid-phase slurry reactors promoted with aluminum chloride catalysts or by vapor-phase reaction of benzene with dilute ethylene-containing feedstock with a boron trifluoride catalyst supported on alumina (Cannella 2007; Clayton and Clayton 1981; HSDB 2009; Welch et al. 2005; Ransley 1984). Newer versions of the method employ synthetic zeolites in fixed-bed reactors as catalysts for alkylation in the liquid phase or narrow pore synthetic zeolites in fixed-bed reactors in the vapor phase (Welch et al. 2005). Other methods of manufacturing ethylbenzene include preparation from acetophenone, dehydrogenation of naphthenes, catalytic cyclization and aromatization, separation from mixed xylenes via fractionation, reaction of ethylmagnesium bromide and chlorobenzene, extraction from coal oil, and recovery from benzene-toluene-xylene (BTX) processing(Clayton and Clayton 1981; HSDB 2009; Ransley 1984; Welch et al. 2005). Commercial grades of ethylbenzene may contain small amounts of m-xylene, p-xylene, cumene, and toluene (HSDB 2009). Ethylbenzene is traditionally ranked as one of the top 50 chemicals produced in the United States. Table 5-1 shows the historical production volumes of ethylbenzene from 1983 to 2005 (C&EN 1994a, 1994b, 1995, 2006; Kirschner 1995). Table 5-2 lists the facilities in each state that manufacture or process ethylbenzene, the intended use, and the range of maximum amounts of ethylbenzene that are stored on site. There are currently 3,755 facilities that produce, process, or use ethylbenzene in the United States. The data listed in Table 5-2 are derived from the Toxics Release Inventory (TRI06 2008). -

Sds – Safety Data Sheet
Effective Date: 02/05/16 Replaces Revision: 01/01/13, 02/26/09 NON-EMERGENCY TELEPHONE 24-HOUR CHEMTREC EMERGENCY TELEPHONE 610-866-4225 800-424-9300 SDS – SAFETY DATA SHEET 1. Identification Product Identifier: ACETONE / ISOPROPYL ALCOHOL BLEND Synonyms: None Chemical Formula: Not Applicable to mixtures Recommended Use of the Chemical and Restrictions On Use: Laboratory Reagent Manufacturer / Supplier: Puritan Products; 2290 Avenue A, Bethlehem, PA 18017 Phone: 610-866-4225 Emergency Phone Number: 24-Hour Chemtrec Emergency Telephone 800-424-9300 2. Hazard(s) Identification Classification of the Substance or Mixture: Flammable liquids (Category 2) Skin irritation (Category 3) Eye irritation (Category 2A) Specific target organ toxicity - single exposure (Category 3) Risk Phrases: R11: Highly flammable. R36: Irritating to eyes. R66: Repeated exposure may cause skin dryness or cracking. R67: Vapors may cause drowsiness and dizziness. Label Elements: Trade Name: ACETONE / ISOPROPYL ALCOHOL BLEND Signal Word: Danger Hazard Statements: H225: Highly flammable liquid and vapor. H316: Causes mild skin irritation. H319: Causes serious eye irritation. H336: May cause drowsiness or dizziness. ACETONE / ISOPROPYL ALCOHOL BLEND Page 1 of 6 Precautionary Statements: P210: Keep away from heat/sparks/open flames/hot surfaces. No smoking. P261: Avoid breathing dust / fume / gas / mist / vapors / spray. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. 3. Composition / Information on Ingredients CAS Number: Not Applicable to mixtures Molecular Weight: Not Applicable to mixtures Ingredient CAS Number EC Number Percent Hazardous Chemical Characterization Acetone 67 - 64 - 1 200-662-2 70 - 90% Yes Substance Isopropyl Alcohol 67 - 63 - 0 200-661-7 10 - 30% Yes Substance 4. -

SAFETY DATA SHEET Acetone
SAFETY DATA SHEET Acetone Revision date: 12.05.15 SDS/004/8 Page 1 of 6 1. Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Acetone EC No. 200-662-2 1.2. Relevant identified uses of the substance or mixture and uses advised against Household Solvent 1.3. Details of the supplier of the safety data sheet Thornton & Ross Ltd, Linthwaite, Huddersfield, HD7 5QH Tel: 01484 842217 Fax: 01484 847301 Email: [email protected] 1.4 Emergency telephone number: Out of normal working hours: +44 870 8510207 2. Hazards identification 2.1. Classification of the substance or mixture According to Regulation EC 1272/2008 classified as Flammable Liquid Category 2, Eye Irritant Category 2, Specific Target Organ Toxicity Single Exposure Category 3. 2.2. Label element GHS Pictogram Signal Word Hazard Class Danger Flammable Liquids, Category 2 Eye Irritation, Category 2 Specific Target Organ Toxicity-Single Exposure, Category 3 Hazard Statements Precautionary statements H225: Highly flammable liquid and vapour. P210: Keep away from heat/sparks/open flames/hot surfaces – No smoking. H319: Causes serious eye irritation P305+351+338: IF IN EYES: Rinse cautiously with H336: May cause drowsiness or dizziness water for several minutes. Remove contact lenses if present and easy to do – continue rinsing. P337+313: Get medical advice/attention. P403: Store in a well ventilated place. Supplemental Hazard Information (EU) EUH066: Repeated exposure may cause skin dryness or cracking 2.3. Other hazards N/A SAFETY DATA SHEET Acetone Revision date: 12.05.15 SDS/004/8 Page 2 of 6 3. -

Toxicological Profile for Acetone Draft for Public Comment
ACETONE 1 Toxicological Profile for Acetone Draft for Public Comment July 2021 ***DRAFT FOR PUBLIC COMMENT*** ACETONE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. This information is distributed solely for the purpose of pre dissemination public comment under applicable information quality guidelines. It has not been formally disseminated by the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy. ***DRAFT FOR PUBLIC COMMENT*** ACETONE iii FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. -

Annexes To: CPMP/ICH/283/95 Impurities: Guideline for Residual Solvents & CVMP/VICH/502/99 Guideline on Impurities
20 February 2013 CPMP/QWP/450/03 -Rev.1, EMEA/CVMP/511/03 -Rev.1 Committee for medicinal products for human use (CHMP) Committee for medicinal products for veterinary use (CVMP) Annexes to: CPMP/ICH/283/95 Impurities: Guideline for residual solvents & CVMP/VICH/502/99 Guideline on impurities: residual solvents Annex I: specifications for class 1 and class 2 residual solvents in active substances Annex II: residues of solvents used in the manufacture of finished products Discussion at Quality Working Party January 2003 to June 2004 Adoption by CVMP July 2004 Adoption by CHMP July 2004 Date for coming into operation January 2005 Rev. 01 Adoption by Quality Working Party 22 November 2012 Rev. 01 Adoption by CVMP 7 February 2013 Rev. 01 Adoption by CHMP 11 February 2013 Rev. 01 Date for coming into operation 1 March 2013 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8416 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Annexes to: CPMP/ICH/283/95 Impurities: Guideline for residual solvents & CVMP/VICH/502/99 Guideline on impurities: residual solvents Introduction The two (V)ICH residual solvents guidelines, ICH Q3C Impurities: Guideline for residual solvents (CPMP/ICH/283/95) and VICH GL18 Guideline on impurities: residual solvents in new veterinary medicinal products, active substances and excipients (CVMP/VICH/502/99), have been in operation for several years, since March 1998 and June 2001 respectively. -

Acetone - Toxfaqs™
Acetone - ToxFAQs™ What is acetone? Acetone is a (manmade) chemical that can also be found in the environment. It is a colorless liquid with a distinct smell and taste. It evaporates easily in air, is flammable, and dissolves in water. Acetone is used by humans to dissolve other substances and to produce plastics, paints and coatings, cleaning products, and personal care products. Other manmade sources of acetone are vehicle exhaust, tobacco smoke, and landfills. Acetone is also released naturally by plants, trees, insects, microbes (germs), volcanic erruptions, and forest fires, and can be found naturally in many fruits and vegetables. Low levels of acetone are produced naturally by the human body, and some health conditions can cause these levels to increase. What happens to acetone in the environment? • Most acetone in the environment exists as vapor in the air, and it can travel long distances this way. • About half of the total acetone in air is broken down by sunlight or other chemicals within 22 days. • Acetone moves from the air into water and soil by rain and snow, and moves quickly from water and soil back into the air. It does not bind to soil or build up in animals. • Acetone can enter surface water as manufacturing waste and seep into groundwater from landfills. • Acetone is broken down by microbes or chemicals in water and soil. How can I be exposed to acetone? A strong scent of acetone • Low levels of acetone are in the air, so most people are exposed and irritation in your eyes, to very small amounts through breathing, but these are rarely at nose, and throat are levels that are harmful to your health. -
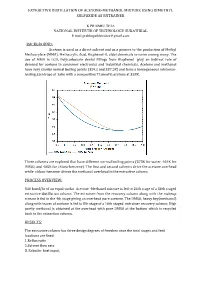
Extractive Distillation of Acetone-Methanol Mixture Using Dimethyl Sulfoxide As Entrainer
EXTRACTIVE DISTILLATION OF ACETONE-METHANOL MIXTURE USING DIMETHYL SULFOXIDE AS ENTRAINER K PRABHU TEJA NATIONAL INSTITUTE OF TECHNOLOGY SURATHKAL E mail:[email protected] BACKGROUND: Acetone is used as a direct solvent and as a pioneer to the production of Methyl Methacrylate (MMA), Methacrylic Acid, Bisphenol-A, aldol chemicals to name among many. The use of MMA in LCD, Polycarbonate dental fillings from Bisphenol play an indirect role of demand for acetone in consumer electronics and industrial chemicals. Acetone and methanol have very similar normal boiling points (329.2 and 337.5K) and form a homogeneous minimum- boiling azeotrope at 1atm with a composition77.6mol% acetone at 328K. Three solvents are explored that have different normal boiling points (373K for water, 464K for DMSO, and 405K for chloro-benzene). The rst and second solvents drive the acetone overhead while chloro-benzene drives the methanol overhead in the extractive column. fi PROCESS OVERVIEW: 540 kmol/hr of an equal-molar Acetone -Methanol mixture is fed to 24th stage of a 36th staged extractive distillation column. The entrainer from the recovery column along with the makeup stream is fed to the 4th stage giving an overhead pure acetone. The DMSO, heavy key(methanol) along with traces of acetone is fed to 8th stage of a 16th staged entrainer recovery column. High purity methanol is obtained at the overhead with pure DMSO at the bottom which is recycled back to the extraction column. RESULTS: The extractive column has three design degrees of freedom once the total stages and feed locations are fixed: 3..Reflux Reboiler ratio heat input. -

Process for Preparing Phenol and Acetone from Cumene
Europaisches Patentamt 0 1 64 568 J) European Patent Office Publication number: B1 Office europeen des brevets EUROPEAN PATENT SPECIFICATION Date of publication of patent specification: 06.07.88 (g) int. ci.4: C 07 C 45/53, C 07 C 37/08, C 07 C 27/00, C 07 C 39/04, Application number: 85105584.8 C 07 C 49/08 Date of filing: 07.05.85 Process for preparing phenol and acetone from cumene. (§) Priority: 24.05.84 US 614124 Proprietor: GENERAL ELECTRIC COMPANY 1 River Road Schenectady New York 12305 (US) Date of publication of application: 18.12.85 Bulletin 85/51 Inventor: Fulmer, John William 78 Park Ridge Drive Publication of the grant of the patent: Mt. Vernon Indiana 47620 (US) 06.07.88 Bulletin 88/27 Representative: Catherine, Alain Designated Contracting States: General Electric France Service de Propriete DEFRGBIT Industrielle 18 Rue Horace Vernet B.P. 76 F-92134 Issy-les-Moulineaux Cedex (FR) References cited: EP-A-0 008 869 EP-A-0 028 522 EP-A-0028910 EP-A-0 032 255 AT-B-205490 AT-B- 219 029 00 DE-A-2 756026 DE-A-3 222 533 US-A-2715145 US-A-4480134 <0 Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall shall be deemed have been filed until the opposition fee has been Q. be filed in a written reasoned statement.