Gas Compatibility | Specialty Gases | Air Liquide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kinetic Modeling for PAH Formation in a Counterflow Diffusion Flame of Isobutane
27th ICDERS July 28th – August 2nd, 2019 Beijing, China Kinetic modeling for PAH formation in a counterflow diffusion flame of isobutane Wei-Kai Chiua, Hairong Taob, Kuang C. Linc,* aDepartment of Mechanical and Electro-Mechanical Engineering,Kaohsiung 80424, National Sun Yat-Sen University, Taiwan bCollege of Chemistry, Beijing Normal University, Beijing 100875, China cEngineering and System Science,Hsinchu 30013, National Tsing Hua University, Taiwan 1 Abstract In the purpose of understanding the complex chemistry of aromatic hydrocarbon formation in diffusion flames of isobutane oxidation, mechanism that consists of 300 species and 11790 reactions is employed for verifying experimental measurements. The kinetic mechanism is incorporated into a 1-D axisymmetric laminar finite-rate model of a counterflow flame to compute the species profiles. Furthermore, the reaction pathway diagram produced by rate of production analysis illustrates the correlation between the decomposition of isobutane and formation of the target species. Keywords: isobutane, counterflow diffusion flame, paromatic hydrocarbon, chemical kinetic modeling 2 Introduction Isobutane (iC4H10) is an isomer of butane and flammable gas. Although it is not ideal fuel on its own, this species plays a role in the oxidation of large hydrocarbon fuels and is used in liquified natural gas (LNG) [1]. The detailed kinetic mechanism of isobutane combustion contains dozens of species and hundreds of reactions. When a kinetic mechanism is applied to a reaction source term of species transport equations, the computational solutions can be used to describe reactive flows and species distribution in multidimensional reactors. This kind of numerical modeling permits designers and analysts to evaluate emissions and performance of fuels in reactors or combustors. -

Toxicological Profile for Hydrogen Sulfide and Carbonyl Sulfide
HYDROGEN SULFIDE AND CARBONYL SULFIDE 149 6. POTENTIAL FOR HUMAN EXPOSURE 6.1 OVERVIEW Hydrogen sulfide has been found in at least 34 of the 1,832 waste sites that have been proposed for inclusion on the EPA National Priorities List (NPL) and carbonyl sulfide was detected in at least 4 of the 1,832 waste sites (ATSDR 2015). However, the number of sites evaluated for these substances is not known and hydrogen sulfide and carbonyl sulfide are ubiquitous in the atmosphere. The frequency of these sites can be seen in Figures 6-1 and 6-2. Carbonyl sulfide and hydrogen sulfide are principal components in the natural sulfur cycle. Bacteria, fungi, and actinomycetes (a fungus-like bacteria) release hydrogen sulfide during the decomposition of 2- sulfur containing proteins and by the direct reduction of sulfate (SO4 ). Hydrogen sulfide is also emitted from volcanoes, stagnant or polluted waters, and manure or coal pits with low oxygen content (Aneja 1990; Khalil and Ramussen 1984). The majority of carbonyl sulfide that enters the environment is released to air and it is very abundant in the troposphere (Conrad and Meuser 2000; EPA 1994c, 1994d; Meinrat et al. 1992; Simmons et al. 2012; Stimler et al. 2010). It enters the atmosphere from both natural and anthropogenic sources (EPA 1994c, 1994d; Meinrat et al. 1992; Stimler et al. 2010). Carbonyl sulfide is released from wetlands, salt marshes, soil, oceans, deciduous and coniferous trees, and volcanic gases (Blake et al. 2004; EPA 1994c, 1994d; Meinrat et al. 1992; Rasmussen et al. 1982a, 1982b; Stimler et al. -

SAFETY DATA SHEET Carbonyl Sulfide
SAFETY DATA SHEET Carbonyl Sulfide Section 1. Identification GHS product identifier : Carbonyl Sulfide Chemical name : carbonyl sulphide Other means of : Carbon oxide sulfide; Carbonyl sulfide; Carbon oxide sulphide (carbonyl sulphide); identification Carbon oxide sulfide (COS); carbon oxysulfide Product use : Synthetic/Analytical chemistry. Synonym : Carbon oxide sulfide; Carbonyl sulfide; Carbon oxide sulphide (carbonyl sulphide); Carbon oxide sulfide (COS); carbon oxysulfide SDS # : 001012 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE GASES - Category 1 substance or mixture GASES UNDER PRESSURE - Liquefied gas ACUTE TOXICITY (inhalation) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : Extremely flammable gas. May form explosive mixtures with air. Contains gas under pressure; may explode if heated. May cause frostbite. Toxic if inhaled. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Always keep container in upright position. Do not depend on odor to detect presence of gas. -

2020 Stainless Steels in Ammonia Production
STAINLESS STEELS IN AMMONIA PRODUCTION A DESIGNERS’ HANDBOOK SERIES NO 9013 Produced by Distributed by AMERICAN IRON NICKEL AND STEEL INSTITUTE INSTITUTE STAINLESS STEELS IN AMMONIA PRODUCTION A DESIGNERS’ HANDBOOK SERIES NO 9013 Originally, this handbook was published in 1978 by the Committee of Stainless Steel Producers, American Iron and Steel Institute. The Nickel Institute republished the handbook in 2020. Despite the age of this publication the information herein is considered to be generally valid. Material presented in the handbook has been prepared for the general information of the reader and should not be used or relied on for specific applications without first securing competent advice. The Nickel Institute, the American Iron and Steel Institute, their members, staff and consultants do not represent or warrant its suitability for any general or specific use and assume no liability or responsibility of any kind in connection with the information herein. Nickel Institute [email protected] www.nickelinstitute.org CONTENTS INTRODUCTION ............................ 4 PROCESS DESCRIPTION ............ 5 CORROSIVES IN AMMONIA PROCESSES ............... 5 CONSIDERATIONS FOR SELECTING STAINLESS STEELS .......................................... 6 Desulfurization of Natural Gas ....................... 6 Catalytic Steam Reforming of Natural Gas ....................... 6 Carbon Monoxide Shift .............. 8 Removal of Carbon Dioxide . 10 Methanation ............................. 11 Synthesis of Ammonia ............. 11 -

Emissions from Small-Scale Combustion of Biomass Fuels - Extensive Quantification and Characterization
Emissions from small-scale combustion of biomass fuels - extensive quantification and characterization Christoffer Boman Anders Nordin Marcus Öhman Dan Boström Energy Technology and Thermal Process Chemistry Umeå University Roger Westerholm Analytical Chemistry, Arrhenius Laboratory Stockholm University _______________________________________________________________ Emissions from small-scale combustion of biomass fuels - extensive quantification and characterization Christoffer Boman1, Anders Nordin1, Roger Westerholm2, Marcus Öhman1, Dan Boström1 1Energy Technology and Thermal Process Chemistry, Umeå University, SE-901 87 Umeå, Sweden 2Analytical Chemistry, Arrhenius Laboratory, Stockholm University, SE-106 91 Stockholm, Sweden STEM-BHM (P12648-1 and P21906-1) Umeå, February 2005 SUMMARY This work was a part of the Swedish national research program concerning emissions and air quality ("Utsläpp och Luftkvalitet") with the sub-programme concerning biomass, health and environment ("Biobränlen, Hälsa, Miljö" - BHM). The main objective of the work was to systematically determine the quantities and characteristics of gaseous and particulate emissions from combustion in residential wood log and biomass fuel pellet appliances and report emission factors for the most important emission components. The specific focus was on present commercial wood and pellet stoves as well as to illustrate the potentials for future technology development. The work was divided in different sub- projects; 1) a literature review of health effects of ambient wood -

Sds – Safety Data Sheet
Effective Date: 02/05/16 Replaces Revision: 01/01/13, 02/26/09 NON-EMERGENCY TELEPHONE 24-HOUR CHEMTREC EMERGENCY TELEPHONE 610-866-4225 800-424-9300 SDS – SAFETY DATA SHEET 1. Identification Product Identifier: ACETONE / ISOPROPYL ALCOHOL BLEND Synonyms: None Chemical Formula: Not Applicable to mixtures Recommended Use of the Chemical and Restrictions On Use: Laboratory Reagent Manufacturer / Supplier: Puritan Products; 2290 Avenue A, Bethlehem, PA 18017 Phone: 610-866-4225 Emergency Phone Number: 24-Hour Chemtrec Emergency Telephone 800-424-9300 2. Hazard(s) Identification Classification of the Substance or Mixture: Flammable liquids (Category 2) Skin irritation (Category 3) Eye irritation (Category 2A) Specific target organ toxicity - single exposure (Category 3) Risk Phrases: R11: Highly flammable. R36: Irritating to eyes. R66: Repeated exposure may cause skin dryness or cracking. R67: Vapors may cause drowsiness and dizziness. Label Elements: Trade Name: ACETONE / ISOPROPYL ALCOHOL BLEND Signal Word: Danger Hazard Statements: H225: Highly flammable liquid and vapor. H316: Causes mild skin irritation. H319: Causes serious eye irritation. H336: May cause drowsiness or dizziness. ACETONE / ISOPROPYL ALCOHOL BLEND Page 1 of 6 Precautionary Statements: P210: Keep away from heat/sparks/open flames/hot surfaces. No smoking. P261: Avoid breathing dust / fume / gas / mist / vapors / spray. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. 3. Composition / Information on Ingredients CAS Number: Not Applicable to mixtures Molecular Weight: Not Applicable to mixtures Ingredient CAS Number EC Number Percent Hazardous Chemical Characterization Acetone 67 - 64 - 1 200-662-2 70 - 90% Yes Substance Isopropyl Alcohol 67 - 63 - 0 200-661-7 10 - 30% Yes Substance 4. -

Carbonyl Sulfide, Dimethyl Sulfide and Carbon Disulfide In
Atmospheric Environment 44 (2010) 3805e3813 Contents lists available at ScienceDirect Atmospheric Environment journal homepage: www.elsevier.com/locate/atmosenv Carbonyl sulfide, dimethyl sulfide and carbon disulfide in the Pearl River Delta of southern China: Impact of anthropogenic and biogenic sources H. Guo a,*, I.J. Simpson b, A.J. Ding c,T.Wanga, S.M. Saunders d, T.J. Wang c, H.R. Cheng a, B. Barletta b, S. Meinardi b, D.R. Blake b, F.S. Rowland b a Department of Civil and Structural Engineering, Hong Kong Polytechnic University, Hong Kong b Department of Chemistry, University of California at Irvine, Irvine, USA c School of Atmospheric Sciences, Nanjing University, China d School of Biomedical, Biomolecular and Chemical Sciences, University of Western Australia, Perth, Australia article info abstract Article history: Reduced sulfur compounds (RSCs) such as carbonyl sulfide (OCS), dimethyl sulfide (DMS) and carbon Received 28 October 2009 disulfide (CS2) impact radiative forcing, ozone depletion, and acid rain. Although Asia is a large source of Received in revised form these compounds, until now a long-term study of their emission patterns has not been carried out. Here 19 June 2010 we analyze 16 months of RSC data measured at a polluted rural/coastal site in the greater Pearl River Accepted 22 June 2010 Delta (PRD) of southern China. A total of 188 canister air samples were collected from August 2001 to December 2002. The OCS and CS2 mixing ratios within these samples were higher in autumn/winter and Keywords: lower in summer due to the influence of Asian monsoon circulations. -

SAFETY DATA SHEET Acetone
SAFETY DATA SHEET Acetone Revision date: 12.05.15 SDS/004/8 Page 1 of 6 1. Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Acetone EC No. 200-662-2 1.2. Relevant identified uses of the substance or mixture and uses advised against Household Solvent 1.3. Details of the supplier of the safety data sheet Thornton & Ross Ltd, Linthwaite, Huddersfield, HD7 5QH Tel: 01484 842217 Fax: 01484 847301 Email: [email protected] 1.4 Emergency telephone number: Out of normal working hours: +44 870 8510207 2. Hazards identification 2.1. Classification of the substance or mixture According to Regulation EC 1272/2008 classified as Flammable Liquid Category 2, Eye Irritant Category 2, Specific Target Organ Toxicity Single Exposure Category 3. 2.2. Label element GHS Pictogram Signal Word Hazard Class Danger Flammable Liquids, Category 2 Eye Irritation, Category 2 Specific Target Organ Toxicity-Single Exposure, Category 3 Hazard Statements Precautionary statements H225: Highly flammable liquid and vapour. P210: Keep away from heat/sparks/open flames/hot surfaces – No smoking. H319: Causes serious eye irritation P305+351+338: IF IN EYES: Rinse cautiously with H336: May cause drowsiness or dizziness water for several minutes. Remove contact lenses if present and easy to do – continue rinsing. P337+313: Get medical advice/attention. P403: Store in a well ventilated place. Supplemental Hazard Information (EU) EUH066: Repeated exposure may cause skin dryness or cracking 2.3. Other hazards N/A SAFETY DATA SHEET Acetone Revision date: 12.05.15 SDS/004/8 Page 2 of 6 3. -

Toxicological Profile for Acetone Draft for Public Comment
ACETONE 1 Toxicological Profile for Acetone Draft for Public Comment July 2021 ***DRAFT FOR PUBLIC COMMENT*** ACETONE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. This information is distributed solely for the purpose of pre dissemination public comment under applicable information quality guidelines. It has not been formally disseminated by the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy. ***DRAFT FOR PUBLIC COMMENT*** ACETONE iii FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. -

Annexes To: CPMP/ICH/283/95 Impurities: Guideline for Residual Solvents & CVMP/VICH/502/99 Guideline on Impurities
20 February 2013 CPMP/QWP/450/03 -Rev.1, EMEA/CVMP/511/03 -Rev.1 Committee for medicinal products for human use (CHMP) Committee for medicinal products for veterinary use (CVMP) Annexes to: CPMP/ICH/283/95 Impurities: Guideline for residual solvents & CVMP/VICH/502/99 Guideline on impurities: residual solvents Annex I: specifications for class 1 and class 2 residual solvents in active substances Annex II: residues of solvents used in the manufacture of finished products Discussion at Quality Working Party January 2003 to June 2004 Adoption by CVMP July 2004 Adoption by CHMP July 2004 Date for coming into operation January 2005 Rev. 01 Adoption by Quality Working Party 22 November 2012 Rev. 01 Adoption by CVMP 7 February 2013 Rev. 01 Adoption by CHMP 11 February 2013 Rev. 01 Date for coming into operation 1 March 2013 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8416 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Annexes to: CPMP/ICH/283/95 Impurities: Guideline for residual solvents & CVMP/VICH/502/99 Guideline on impurities: residual solvents Introduction The two (V)ICH residual solvents guidelines, ICH Q3C Impurities: Guideline for residual solvents (CPMP/ICH/283/95) and VICH GL18 Guideline on impurities: residual solvents in new veterinary medicinal products, active substances and excipients (CVMP/VICH/502/99), have been in operation for several years, since March 1998 and June 2001 respectively. -

Acetone - Toxfaqs™
Acetone - ToxFAQs™ What is acetone? Acetone is a (manmade) chemical that can also be found in the environment. It is a colorless liquid with a distinct smell and taste. It evaporates easily in air, is flammable, and dissolves in water. Acetone is used by humans to dissolve other substances and to produce plastics, paints and coatings, cleaning products, and personal care products. Other manmade sources of acetone are vehicle exhaust, tobacco smoke, and landfills. Acetone is also released naturally by plants, trees, insects, microbes (germs), volcanic erruptions, and forest fires, and can be found naturally in many fruits and vegetables. Low levels of acetone are produced naturally by the human body, and some health conditions can cause these levels to increase. What happens to acetone in the environment? • Most acetone in the environment exists as vapor in the air, and it can travel long distances this way. • About half of the total acetone in air is broken down by sunlight or other chemicals within 22 days. • Acetone moves from the air into water and soil by rain and snow, and moves quickly from water and soil back into the air. It does not bind to soil or build up in animals. • Acetone can enter surface water as manufacturing waste and seep into groundwater from landfills. • Acetone is broken down by microbes or chemicals in water and soil. How can I be exposed to acetone? A strong scent of acetone • Low levels of acetone are in the air, so most people are exposed and irritation in your eyes, to very small amounts through breathing, but these are rarely at nose, and throat are levels that are harmful to your health. -
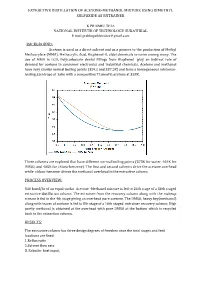
Extractive Distillation of Acetone-Methanol Mixture Using Dimethyl Sulfoxide As Entrainer
EXTRACTIVE DISTILLATION OF ACETONE-METHANOL MIXTURE USING DIMETHYL SULFOXIDE AS ENTRAINER K PRABHU TEJA NATIONAL INSTITUTE OF TECHNOLOGY SURATHKAL E mail:[email protected] BACKGROUND: Acetone is used as a direct solvent and as a pioneer to the production of Methyl Methacrylate (MMA), Methacrylic Acid, Bisphenol-A, aldol chemicals to name among many. The use of MMA in LCD, Polycarbonate dental fillings from Bisphenol play an indirect role of demand for acetone in consumer electronics and industrial chemicals. Acetone and methanol have very similar normal boiling points (329.2 and 337.5K) and form a homogeneous minimum- boiling azeotrope at 1atm with a composition77.6mol% acetone at 328K. Three solvents are explored that have different normal boiling points (373K for water, 464K for DMSO, and 405K for chloro-benzene). The rst and second solvents drive the acetone overhead while chloro-benzene drives the methanol overhead in the extractive column. fi PROCESS OVERVIEW: 540 kmol/hr of an equal-molar Acetone -Methanol mixture is fed to 24th stage of a 36th staged extractive distillation column. The entrainer from the recovery column along with the makeup stream is fed to the 4th stage giving an overhead pure acetone. The DMSO, heavy key(methanol) along with traces of acetone is fed to 8th stage of a 16th staged entrainer recovery column. High purity methanol is obtained at the overhead with pure DMSO at the bottom which is recycled back to the extraction column. RESULTS: The extractive column has three design degrees of freedom once the total stages and feed locations are fixed: 3..Reflux Reboiler ratio heat input.