SAFETY DATA SHEET Acetone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Experimental and Modeling Study of the Flammability of Fuel Tank DE-AC36-08-GO28308 Headspace Vapors from High Ethanol Content Fuels 5B
An Experimental and Modeling Subcontract Report NREL/SR-540-44040 Study of the Flammability of October 2008 Fuel Tank Headspace Vapors from High Ethanol Content Fuels D. Gardiner, M. Bardon, and G. Pucher Nexum Research Corporation Mallorytown, K0E 1R0, Canada An Experimental and Modeling Subcontract Report NREL/SR-540-44040 Study of the Flammability of October 2008 Fuel Tank Headspace Vapors from High Ethanol Content Fuels D. Gardiner, M. Bardon, and G. Pucher Nexum Research Corporation Mallorytown, K0E 1R0, Canada NREL Technical Monitor: M. Melendez Prepared under Subcontract No. XCI-5-55505-01 Period of Performance: August 2005 – December 2008 National Renewable Energy Laboratory 1617 Cole Boulevard, Golden, Colorado 80401-3393 303-275-3000 • www.nrel.gov NREL is a national laboratory of the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Operated by the Alliance for Sustainable Energy, LLC Contract No. DE-AC36-08-GO28308 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. -

Liquefied Natural Gas Deerfoot Consulting Inc. Liquefied Natural Gas Liquefied Methane; LNG. Fuel. Not Available. Fortisbc 16705
Liquefied Natural Gas SAFETY DATA SHEET Date of Preparation: January 7, 2019 Section 1: IDENTIFICATION Product Name: Liquefied Natural Gas Synonyms: Liquefied Methane; LNG. Product Use: Fuel. Restrictions on Use: Not available. Manufacturer/Supplier: FortisBC 16705 Fraser Highway Surrey, BC V3S 2X7 Phone Number: Tilbury: (604) 946-4818 , Mt. Hayes: (236) 933-2060 Emergency Phone: FortisBC Gas Emergency: 1-800-663-9911 LNG Transportation Emergency: 1-877-889-2002 Date of Preparation of SDS: January 7, 2019 Section 2: HAZARD(S) IDENTIFICATION GHS INFORMATION Classification: Flammable Gases, Category 1 Gases Under Pressure - Refrigerated Liquefied Gas Simple Asphyxiant, Category 1 LABEL ELEMENTS Hazard Pictogram(s): Signal Word: Danger Hazard Extremely flammable gas. Statements: Contains refrigerated gas; may cause cryogenic burns or injury. May displace oxygen and cause rapid suffocation. Precautionary Statements Prevention: Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Wear cold insulating gloves and either face shield or eye protection. Response: Get immediate medical advice/attention. Thaw frosted parts with lukewarm water. Do not rub affected area. Leaking gas fire: Do not extinguish, unless leak can be stopped safely. In case of leakage, eliminate all ignition sources. Storage: Store in a well-ventilated place. Disposal: Not applicable. Hazards Not Otherwise Classified: Not applicable. Ingredients with Unknown Toxicity: None. This material is considered hazardous by the OSHA Hazard Communication Standard, (29 CFR 1910.1200). This material is considered hazardous by the Hazardous Products Regulations. Page 1 of 11 Deerfoot Consulting Inc. Liquefied Natural Gas SAFETY DATA SHEET Date of Preparation: January 7, 2019 Section 3: COMPOSITION / INFORMATION ON INGREDIENTS Hazardous Ingredient(s) Common name / CAS No. -

Sds – Safety Data Sheet
Effective Date: 02/05/16 Replaces Revision: 01/01/13, 02/26/09 NON-EMERGENCY TELEPHONE 24-HOUR CHEMTREC EMERGENCY TELEPHONE 610-866-4225 800-424-9300 SDS – SAFETY DATA SHEET 1. Identification Product Identifier: ACETONE / ISOPROPYL ALCOHOL BLEND Synonyms: None Chemical Formula: Not Applicable to mixtures Recommended Use of the Chemical and Restrictions On Use: Laboratory Reagent Manufacturer / Supplier: Puritan Products; 2290 Avenue A, Bethlehem, PA 18017 Phone: 610-866-4225 Emergency Phone Number: 24-Hour Chemtrec Emergency Telephone 800-424-9300 2. Hazard(s) Identification Classification of the Substance or Mixture: Flammable liquids (Category 2) Skin irritation (Category 3) Eye irritation (Category 2A) Specific target organ toxicity - single exposure (Category 3) Risk Phrases: R11: Highly flammable. R36: Irritating to eyes. R66: Repeated exposure may cause skin dryness or cracking. R67: Vapors may cause drowsiness and dizziness. Label Elements: Trade Name: ACETONE / ISOPROPYL ALCOHOL BLEND Signal Word: Danger Hazard Statements: H225: Highly flammable liquid and vapor. H316: Causes mild skin irritation. H319: Causes serious eye irritation. H336: May cause drowsiness or dizziness. ACETONE / ISOPROPYL ALCOHOL BLEND Page 1 of 6 Precautionary Statements: P210: Keep away from heat/sparks/open flames/hot surfaces. No smoking. P261: Avoid breathing dust / fume / gas / mist / vapors / spray. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. 3. Composition / Information on Ingredients CAS Number: Not Applicable to mixtures Molecular Weight: Not Applicable to mixtures Ingredient CAS Number EC Number Percent Hazardous Chemical Characterization Acetone 67 - 64 - 1 200-662-2 70 - 90% Yes Substance Isopropyl Alcohol 67 - 63 - 0 200-661-7 10 - 30% Yes Substance 4. -

Material Safety Data Sheet
SAFETY DATA SHEET EFFECTIVE JUNE 2016 SECTION 1 – PRODUCT & COMPANY IDENTIFICATION Product Name: Commercial Odorized Propane Chemical Name: Propane (C3H8) Chemical Family: Petroleum Hydrocarbon Common Names: Liquefied Petroleum Gas, LP-Gas, LPG, Bottle Gas Intended Use: Propane is a liquid fuel Distributor: Campora Propane Service, PO Box 31717 Stockton, CA 95213 Emergency Response: CHEMTREC (800) 424-9300 General Information: (209) 941-2994 SECTION 2 – CHEMICAL HAZARD CLASSIFICATION & WARNING INFORMATION Fire Hazard NFPA CLASSES: 1-Slight 2-Moderate 3-Serious Health Hazard Reactivity 4-Severe Physical hazards Flammable gases Category 1 Gases under pressure Liquefied gas Health hazards Acute toxicity, inhalation Category 4 Germ cell mutagenicity Category 1B Carcinogenicity Category 1A Reproductive toxicity Category 1A Specific target organ toxicity, repeated Category 2 exposure OSHA defined hazards Not classified. Label Elements Signal Word Danger Hazard Statement Propane (also called LPG-Liquefied Petroleum Gas or LP-Gas) is a liquid fuel stored under pressure. In most systems, propane is vaporized to a gas before it leaves the tank. Propane is highly flammable when mixed with air (oxygen) and can be ignited by many sources, including open flames, smoking materials, electrical sparks, and static electricity. Severe “freeze burn” or frostbite can result if propane liquid comes in contact with your skin. Extremely flammable gas. Harmful if inhaled. May cause genetic defects. May cause cancer. May damage fertility or the unborn child. May cause damage to Blood through prolonged or repeated exposure. May cause cryogenic burns or injury. Propane is a simple asphyxiant. Precautionary statement General Read and follow all Safety Data Sheets (SDS’S) before use. -

Toxicological Profile for Acetone Draft for Public Comment
ACETONE 1 Toxicological Profile for Acetone Draft for Public Comment July 2021 ***DRAFT FOR PUBLIC COMMENT*** ACETONE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. This information is distributed solely for the purpose of pre dissemination public comment under applicable information quality guidelines. It has not been formally disseminated by the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy. ***DRAFT FOR PUBLIC COMMENT*** ACETONE iii FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. -

Univar USA Inc Safety Data Sheet
Univar USA Inc Safety Data Sheet SDS No: Version No: Order No: 3075 Highland Pkwy, Ste 200, Downers Grove, IL 60515 (425) 889 3400 Emergency Assistance For emergency assistance involving chemicals call Chemtrec - (800) 424-9300 UNIVAR USA INC. SDS NO:10000230 ISSUE DATE:2015-05-19 VERSION:001 2015-05-19 Annotation: Univar 3075 Highland Pkwy STE 200 Downers Grove, IL 60515 425-889-3400 SAFETY DATA SHEET 1. Identification Product identifier: AROMATIC 150 (CLASS 3) Other means of identification SDS number: 000100000230 Recommended use and restriction on use Recommended use: Not available. Restrictions on use: Not known. Emergency telephone number:For emergency assistance Involving chemicals call CHEMTREC day or night at: 1-800-424-9300. CHEMTREC INTERNATIONAL Tel# 703-527-3887 2. Hazard(s) identification Hazard classification Physical hazards Flammable liquids Category 4 Health hazards Acute toxicity (Dermal) Category 4 Skin corrosion/irritation Category 2 Carcinogenicity Category 1B Environmental hazardsAcute hazards Category 3 to the aquatic environment Label elements Hazard symbol UNIVAR USA INC. SDS NO:10000230 ISSUE DATE:2015-05-19 VERSION:001 2015-05-19 Annotation: Version: 1.0 Revision date: 05/19/2015 Signal word Danger Hazard statement Combustible liquid. May cause flash fire or explosion. Harmful in contact with skin. Causes skin irritation. May cause cancer. Harmful to aquatic life. Precautionary statement Prevention Wear protective gloves/protective clothing/eye protection/face protection. Wash thoroughly after handling. Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Use personal protective equipment as required. Response IF ON SKIN: Wash with plenty of water. -

Annexes To: CPMP/ICH/283/95 Impurities: Guideline for Residual Solvents & CVMP/VICH/502/99 Guideline on Impurities
20 February 2013 CPMP/QWP/450/03 -Rev.1, EMEA/CVMP/511/03 -Rev.1 Committee for medicinal products for human use (CHMP) Committee for medicinal products for veterinary use (CVMP) Annexes to: CPMP/ICH/283/95 Impurities: Guideline for residual solvents & CVMP/VICH/502/99 Guideline on impurities: residual solvents Annex I: specifications for class 1 and class 2 residual solvents in active substances Annex II: residues of solvents used in the manufacture of finished products Discussion at Quality Working Party January 2003 to June 2004 Adoption by CVMP July 2004 Adoption by CHMP July 2004 Date for coming into operation January 2005 Rev. 01 Adoption by Quality Working Party 22 November 2012 Rev. 01 Adoption by CVMP 7 February 2013 Rev. 01 Adoption by CHMP 11 February 2013 Rev. 01 Date for coming into operation 1 March 2013 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8416 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Annexes to: CPMP/ICH/283/95 Impurities: Guideline for residual solvents & CVMP/VICH/502/99 Guideline on impurities: residual solvents Introduction The two (V)ICH residual solvents guidelines, ICH Q3C Impurities: Guideline for residual solvents (CPMP/ICH/283/95) and VICH GL18 Guideline on impurities: residual solvents in new veterinary medicinal products, active substances and excipients (CVMP/VICH/502/99), have been in operation for several years, since March 1998 and June 2001 respectively. -

Acetone - Toxfaqs™
Acetone - ToxFAQs™ What is acetone? Acetone is a (manmade) chemical that can also be found in the environment. It is a colorless liquid with a distinct smell and taste. It evaporates easily in air, is flammable, and dissolves in water. Acetone is used by humans to dissolve other substances and to produce plastics, paints and coatings, cleaning products, and personal care products. Other manmade sources of acetone are vehicle exhaust, tobacco smoke, and landfills. Acetone is also released naturally by plants, trees, insects, microbes (germs), volcanic erruptions, and forest fires, and can be found naturally in many fruits and vegetables. Low levels of acetone are produced naturally by the human body, and some health conditions can cause these levels to increase. What happens to acetone in the environment? • Most acetone in the environment exists as vapor in the air, and it can travel long distances this way. • About half of the total acetone in air is broken down by sunlight or other chemicals within 22 days. • Acetone moves from the air into water and soil by rain and snow, and moves quickly from water and soil back into the air. It does not bind to soil or build up in animals. • Acetone can enter surface water as manufacturing waste and seep into groundwater from landfills. • Acetone is broken down by microbes or chemicals in water and soil. How can I be exposed to acetone? A strong scent of acetone • Low levels of acetone are in the air, so most people are exposed and irritation in your eyes, to very small amounts through breathing, but these are rarely at nose, and throat are levels that are harmful to your health. -
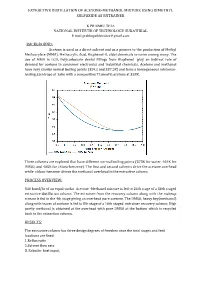
Extractive Distillation of Acetone-Methanol Mixture Using Dimethyl Sulfoxide As Entrainer
EXTRACTIVE DISTILLATION OF ACETONE-METHANOL MIXTURE USING DIMETHYL SULFOXIDE AS ENTRAINER K PRABHU TEJA NATIONAL INSTITUTE OF TECHNOLOGY SURATHKAL E mail:[email protected] BACKGROUND: Acetone is used as a direct solvent and as a pioneer to the production of Methyl Methacrylate (MMA), Methacrylic Acid, Bisphenol-A, aldol chemicals to name among many. The use of MMA in LCD, Polycarbonate dental fillings from Bisphenol play an indirect role of demand for acetone in consumer electronics and industrial chemicals. Acetone and methanol have very similar normal boiling points (329.2 and 337.5K) and form a homogeneous minimum- boiling azeotrope at 1atm with a composition77.6mol% acetone at 328K. Three solvents are explored that have different normal boiling points (373K for water, 464K for DMSO, and 405K for chloro-benzene). The rst and second solvents drive the acetone overhead while chloro-benzene drives the methanol overhead in the extractive column. fi PROCESS OVERVIEW: 540 kmol/hr of an equal-molar Acetone -Methanol mixture is fed to 24th stage of a 36th staged extractive distillation column. The entrainer from the recovery column along with the makeup stream is fed to the 4th stage giving an overhead pure acetone. The DMSO, heavy key(methanol) along with traces of acetone is fed to 8th stage of a 16th staged entrainer recovery column. High purity methanol is obtained at the overhead with pure DMSO at the bottom which is recycled back to the extraction column. RESULTS: The extractive column has three design degrees of freedom once the total stages and feed locations are fixed: 3..Reflux Reboiler ratio heat input. -

An Overview of Hydrogen As a Vehicle Fuel
Renewable and Sustainable Energy Reviews 16 (2012) 5511–5528 Contents lists available at SciVerse ScienceDirect Renewable and Sustainable Energy Reviews journal homepage: www.elsevier.com/locate/rser An overview of hydrogen as a vehicle fuel H. Fayaz a,b,n, R. Saidur a,b, N. Razali a, F.S. Anuar a,b, A.R. Saleman a,b, M.R. Islam b a Department of Mechanical Engineering, University of Malaya, Kuala Lumpur 50603, Malaysia b Centre of Research UMPEDAC, Level 4, Engineering Tower, Faculty of Engineering, University of Malaya, Kuala Lumpur 50603, Malaysia article info abstract Article history: As hydrogen fuel cell vehicles move from manifestation to commercialization, the users expect safe, Received 24 December 2010 convenient and customer-friendly fuelling. Hydrogen quality affects fuel cell stack performance and Received in revised form lifetime, as well as other factors such as valve operation. In this paper, previous researcher’s development 1 June 2012 on hydrogen as a possible major fuel of the future has been studied thoroughly. Hydrogen is one of the Accepted 4 June 2012 energy carriers which can replace fossil fuel and can be used as fuel in an internal combustion engines and as a fuel cell in vehicles. To use hydrogen as a fuel of internal combustion engine, engine design should be Keywords: considered for avoiding abnormal combustion. As a result it can improve engine efficiency, power output Hydrogen and reduce NOx emissions. The emission of fuel cell is low as compared to conventional vehicles but as Vehicle fuel penalty, fuel cell vehicles need additional space and weight to install the battery and storage tank, thus Engine increases it production cost. -

Constants of Explosive Limits
Constants of explosive limits Ali M. Nassimia,∗ Mohammad Jafarib, Hossein Farrokhpoura and Mohammad H. Keshavarzb aDepartment of Chemistry, Isfahan University of Technology, Isfahan 8115683111, Iran bDepartment of Chemistry, Malek Ashtar University of Technology, Shahin Shahr, 83145 115, Iran January 23, 2017 Abstract This work defines density factor as the ratio of before ignition density to after ignition density of the ignition mixture. This work provides an estimation method for explosive limits of various fuels under room temperature and pressure by showing that for a large universe of fuels, constant adiabatic flame temperature and density factor are appropriate approximations at the lower explosive limit while only a constant density factor might be an appropriate approximation at the upper explosive limit. Thus the assumption of a constant adiabatic flame temperature can be used in calculating lower explosive limit while the assumption of a constant density factor can be used in approximating upper explosive limit. Keywords: Explosive limit; Flammability limit; Adiabatic flame temperature; Density factor; LEL, UEL, LFL, UFL. List of abbreviations: Lower explosive limit (LEL), upper explosive limit (UEL), explosive limit (EL), adiabatic flame temperature (AFT), theoretical threshold temperature (TTT), Equivalence ratio (φ), Chemical Equilibrium with Applications (CEA), Constant Enthalpy and Pressure (HP), Constant internal energy and volume (UV), Lower limit flame temperature (LLFT), Upper limit flame temperature (ULFT), Limit flame temperature (LFT), Lower limit density factor (LLDF), Upper limit density factor (ULDF), Limit density factor (LDF). 1 Introduction Flammability parameters are important safety considerations in various applications ranging from the design of combustion chambers to the design of fuel tanks. Moreover, a knowledge of the flammability arXiv:1701.00909v2 [physics.chem-ph] 20 Jan 2017 parameters is used in judging fire and explosion hazards of technological processes and technological installations. -

Hydrogen Flammability Limits and Implications on Fire Safety of Transportation Vehicles
Hydrogen Flammability Limits and Implications on Fire Safety of Transportation Vehicles by Umit O. Koylu A University Transportation Center Program at Missouri University of Science & Technology UTC R170 Disclaimer The contents of this report reflect the views of the author(s), who are responsible for the facts and the accuracy of information presented herein. This document is disseminated under the sponsorship of the Department of Transportation, University Transportation Centers Program and the Center for Infrastructure Engineering Studies UTC program at the University of Missouri - Rolla, in the interest of information exchange. The U.S. Government and Center for Infrastructure Engineering Studies assumes no liability for the contents or use thereof. NUTC ### Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. UTC R170 4. Title and Subtitle 5. Report Date Hydrogen Flammability Limits and Implications on Fire Safety of Transportation Vehicles January 2008 6. Performing Organization Code 7. Author/s 8. Performing Organization Report No. Umit O. Koylu 00013059 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) 11. Contract or Grant No. Center for Infrastructure Engineering Studies/UTC program Missouri University of Science & Technology DTRS98-G-0021 223 Engineering Research Lab Rolla, MO 65409 12. Sponsoring Organization Name and Address 13. Type of Report and Period Covered U.S. Department of Transportation Research and Special Programs Administration Final th 400 7 Street, SW 14. Sponsoring Agency Code Washington, DC 20590-0001 15. Supplementary Notes 16. Abstract The recent establishment of the National University Transportation Center at MST under the "Safe, Accountable, Flexible, Efficient Transportation Equity Act: A Legacy for Users," expands the research and education activities to include alternative transportation fuels and other issues that are at the forefront of society and the national agenda.