United States Patent Office Patented Dec
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

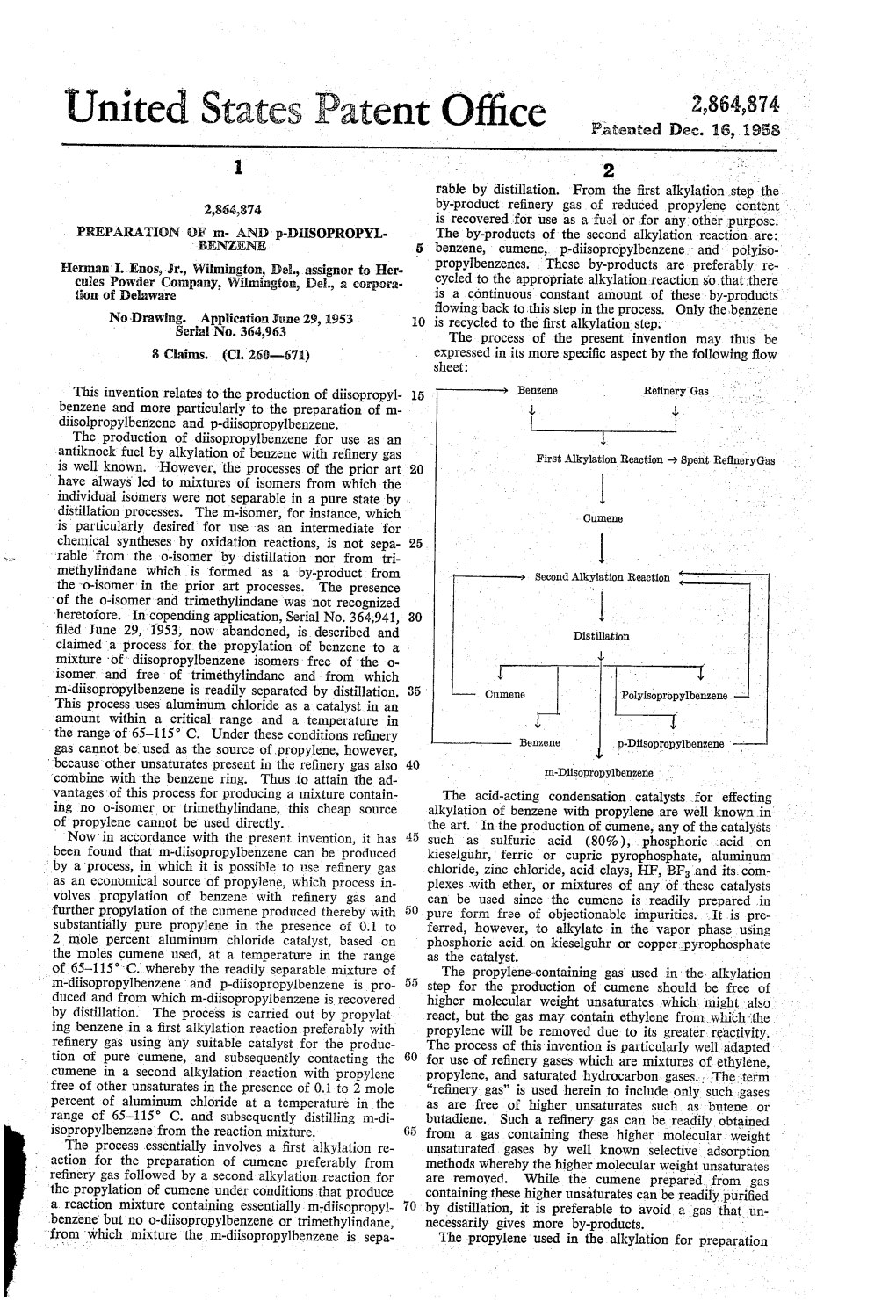
An Improved Process for the Production of Cumene
Europaisches Patentamt European Patent Office © Publication number: 0 537 389 A1 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 91309531.1 int. Ci.5; C07C 2/66, C07C 2/86, C07C 6/12, C07C 15/085 (§) Date of filing: 16.10.91 ® Date of publication of application: @ Applicant: Council of Scientific and Industrial 21.04.93 Bulletin 93/16 Research Rafi Marg @ Designated Contracting States: New Delhi 110 001 (IN) BE DE FR GB IT NL @ Inventor: Pradhan, Ajit Ramchandra National Chemical Laboratory Pune-411008, Maharashtra(IN) Inventor: Rao, Bollapragad Seshagiri National Chemical Laboratory Pune-411008, Maharashtra(IN) 0 Representative: Collier, Jeremy Austin Grey et al J.A.Kemp & Co., 14 South Square, Gray's Inn London WC1R 5LX (GB) 0 An improved process for the production of cumene. © An improved process is disclosed for the preparation of cumene. Cumene is prepared by reacting benzene with a propylating agent in the presence of a catalyst containing metal loaded Zeolite EU-1 in a reactor in the range of a temperature of 150 to 250 °C and a pressure of 1 to 35 atmospheres, the propyl and diisopropylben- zene so formed are separated from the reactor effluent by conventional methods. The diisopropylbenzene is recycled back to the reactor. Simultaneous alkylation and transalkylation reactions occur in a single catalyst bed containing Zeolite EU-1 with a feed containing benzene, propylene and diisopropylbenzene. Cumenes are important chemical precursors in the production of detergents and polymers among others. Oi CO CO IV CO m Rank Xerox (UK) Business Services (3. 10/3.5x/3.0. -

ECO-Ssls for Pahs
Ecological Soil Screening Levels for Polycyclic Aromatic Hydrocarbons (PAHs) Interim Final OSWER Directive 9285.7-78 U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response 1200 Pennsylvania Avenue, N.W. Washington, DC 20460 June 2007 This page intentionally left blank TABLE OF CONTENTS 1.0 INTRODUCTION .......................................................1 2.0 SUMMARY OF ECO-SSLs FOR PAHs......................................1 3.0 ECO-SSL FOR TERRESTRIAL PLANTS....................................4 5.0 ECO-SSL FOR AVIAN WILDLIFE.........................................8 6.0 ECO-SSL FOR MAMMALIAN WILDLIFE..................................8 6.1 Mammalian TRV ...................................................8 6.2 Estimation of Dose and Calculation of the Eco-SSL ........................9 7.0 REFERENCES .........................................................16 7.1 General PAH References ............................................16 7.2 References Used for Derivation of Plant and Soil Invertebrate Eco-SSLs ......17 7.3 References Rejected for Use in Derivation of Plant and Soil Invertebrate Eco-SSLs ...............................................................18 7.4 References Used in Derivation of Wildlife TRVs .........................25 7.5 References Rejected for Use in Derivation of Wildlife TRV ................28 i LIST OF TABLES Table 2.1 PAH Eco-SSLs (mg/kg dry weight in soil) ..............................4 Table 3.1 Plant Toxicity Data - PAHs ..........................................5 Table 4.1 -

Specifications Guide Americas Petrochemicals Latest Update: July 2020
Specifications Guide Americas Petrochemicals Latest update: July 2020 Definitions of the trading locations for which Platts publishes daily indexes or assessments 2 Olefins 3 US aromatics 6 Latin American aromatics 8 US polymers 10 Latin American polymers 13 US intermediates 16 US hydrocarbon solvents 17 US chlor alkali 18 US oxygenated solvents 19 Liquid and gas chemical freight 21 Global petrochemical indices 22 Revision history 23 www.spglobal.com/platts Specifications Guide Americas Petrochemicals: July 2020 DEFINITIONS OF THE TRADING LOCATIONS FOR WHICH PLATTS PUBLISHES DAILY INDEXES OR ASSESSMENTS The following specifications guide contains the primary specifications for S&P Global Platts petrochemical assessments in the Americas. All the assessments listed here employ Platts Assessments Methodology, as published at https://www.spglobal.com/platts/plattscontent/_assets/_files/en/our-methodology/methodology-specifications/platts-assessments-methodology-guide.pdf. These guides are designed to give Platts subscribers as much information as possible about a wide range of methodology and specification questions. This guide is current at the time of publication. Platts may issue further updates and enhancements to this methodology and will announce these to subscribers through its usual publications of record. Such updates will be included in the next version of this guide. Platts editorial staff and managers are available to provide guidance when assessment issues require clarification. OLEFINS Assessment CURRENCY CODE Mavg Wavg TYPE -

CUMENE Cumene Is the Main Feedstock for Phenol/Acetone
CUMENE Cumene is the main feedstock for phenol/acetone production and demand is driven by market growth for the derivatives of phenol and acetone such as bis-phenol A, phenolic resins and caprolactam. Other uses for cumene are as a thinner for paints, lacquers and enamels, and as a constituent of some petroleum-based solvents. It is also used in manufacturing polymerisation catalysts, catalyst for acrylic and polyester type resins, and as a raw material for peroxides and oxidation catalysts. Cumene is considered a primary skin and eye irritant and excessive exposure can lead to headaches and narcosis. Cumene is stable at room temperature but must be kept well away from oxidising agents. It is a fire and explosion hazard and above 31oC explosive vapour/air mixtures may be formed. Cumene is released to the environment as a result of its production and processing, from petroleum refining and the evaporation and combustion of petroleum products and by the use of a variety of products containing cumene. When released to the atmosphere, vapour phase cumene will react with photochemically generated hydroxyl radicals with an estimated half-life of 25 hours in polluted atmospheres and 49 hours in normal atmospheres. Cumene is a contaminant of air, sediments and surface, drinking and groundwater and a natural constituent of a variety of foods and vegetation. ICIS pricing quotes cumene in the USA. Frequency: Published weekly on Fridays. Cumene (USA) Weekly Price Assessments: Cumene Contract Prices FOB monthly (US CTS/LB & conversion to USD/MT): General information: Assessment window: Assessments are based on information supplied by market participants through the week up to 1800 hours in Houston on Fridays. -

Toxicological Profile for Ethylbenzene
ETHYLBENZENE 151 5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL 5.1 PRODUCTION Ethylbenzene is primarily produced by the alkylation of benzene with ethylene in liquid-phase slurry reactors promoted with aluminum chloride catalysts or by vapor-phase reaction of benzene with dilute ethylene-containing feedstock with a boron trifluoride catalyst supported on alumina (Cannella 2007; Clayton and Clayton 1981; HSDB 2009; Welch et al. 2005; Ransley 1984). Newer versions of the method employ synthetic zeolites in fixed-bed reactors as catalysts for alkylation in the liquid phase or narrow pore synthetic zeolites in fixed-bed reactors in the vapor phase (Welch et al. 2005). Other methods of manufacturing ethylbenzene include preparation from acetophenone, dehydrogenation of naphthenes, catalytic cyclization and aromatization, separation from mixed xylenes via fractionation, reaction of ethylmagnesium bromide and chlorobenzene, extraction from coal oil, and recovery from benzene-toluene-xylene (BTX) processing(Clayton and Clayton 1981; HSDB 2009; Ransley 1984; Welch et al. 2005). Commercial grades of ethylbenzene may contain small amounts of m-xylene, p-xylene, cumene, and toluene (HSDB 2009). Ethylbenzene is traditionally ranked as one of the top 50 chemicals produced in the United States. Table 5-1 shows the historical production volumes of ethylbenzene from 1983 to 2005 (C&EN 1994a, 1994b, 1995, 2006; Kirschner 1995). Table 5-2 lists the facilities in each state that manufacture or process ethylbenzene, the intended use, and the range of maximum amounts of ethylbenzene that are stored on site. There are currently 3,755 facilities that produce, process, or use ethylbenzene in the United States. The data listed in Table 5-2 are derived from the Toxics Release Inventory (TRI06 2008). -

Process for Preparing Phenol and Acetone from Cumene
Europaisches Patentamt 0 1 64 568 J) European Patent Office Publication number: B1 Office europeen des brevets EUROPEAN PATENT SPECIFICATION Date of publication of patent specification: 06.07.88 (g) int. ci.4: C 07 C 45/53, C 07 C 37/08, C 07 C 27/00, C 07 C 39/04, Application number: 85105584.8 C 07 C 49/08 Date of filing: 07.05.85 Process for preparing phenol and acetone from cumene. (§) Priority: 24.05.84 US 614124 Proprietor: GENERAL ELECTRIC COMPANY 1 River Road Schenectady New York 12305 (US) Date of publication of application: 18.12.85 Bulletin 85/51 Inventor: Fulmer, John William 78 Park Ridge Drive Publication of the grant of the patent: Mt. Vernon Indiana 47620 (US) 06.07.88 Bulletin 88/27 Representative: Catherine, Alain Designated Contracting States: General Electric France Service de Propriete DEFRGBIT Industrielle 18 Rue Horace Vernet B.P. 76 F-92134 Issy-les-Moulineaux Cedex (FR) References cited: EP-A-0 008 869 EP-A-0 028 522 EP-A-0028910 EP-A-0 032 255 AT-B-205490 AT-B- 219 029 00 DE-A-2 756026 DE-A-3 222 533 US-A-2715145 US-A-4480134 <0 Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall shall be deemed have been filed until the opposition fee has been Q. be filed in a written reasoned statement. -

Marathon Petroleum Cumene
SAFETY DATA SHEET SDS ID NO.: 0243MAR019 Revision Date 05/14/2015 1. IDENTIFICATION Product Name: Marathon Petroleum Cumene Synonym: Cumene; Isopropylbenzene Product Code: 0243MAR019 Chemical Family: Aromatic Hydrocarbon Recommended Use: Solvent. Chemical intermediate. Gasoline blending. Restrictions on Use: All others. Manufacturer, Importer, or Responsible Party Name and Address: MARATHON PETROLEUM COMPANY LP 539 South Main Street Findlay, OH 45840 SDS information: 1-419-421-3070 Emergency Telephone: 1-877-627-5463 2. HAZARD IDENTIFICATION Classification OSHA Regulatory Status This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Flammable liquids Category 3 Carcinogenicity Category 2 Specific target organ toxicity (single exposure) Category 3 Aspiration toxicity Category 1 Acute aquatic toxicity Category 2 Chronic aquatic toxicity Category 3 Hazards Not Otherwise Classified (HNOC) Static accumulating flammable liquid May form explosive peroxides Label elements EMERGENCY OVERVIEW Danger FLAMMABLE LIQUID AND VAPOR May accumulate electrostatic charge and ignite or explode May form explosive peroxides May be fatal if swallowed and enters airways May cause respiratory irritation SDS ID NO.: 0243MAR019 Product name: Marathon Petroleum Cumene Page 1 of 9 0243MAR019 Marathon Petroleum Cumene Revision Date 05/14/2015 _____________________________________________________________________________________________ May cause drowsiness or dizziness Suspected of causing cancer by inhalation Toxic to aquatic life Harmful to aquatic life with long lasting effects Appearance Colorless Liquid Physical State Liquid Odor Aromatic Precautionary Statements - Prevention Obtain special instructions before use Do not handle until all safety precautions have been read and understood Keep away from heat/sparks/open flames/hot surfaces. - No smoking Keep container tightly closed Ground/bond container and receiving equipment Use explosion-proof electrical/ventilating/lighting/equipment Use only non-sparking tools. -

Alpha-Methylstyrene from Dehydrogenation of Cumene Over Fe2o3-Cr2o3-K2CO3 Catalyst
石 油 学 会 誌 Sekiyu Gakkaishi, 32, (2), 87-91 (1989) 87 Alpha-methylstyrene from Dehydrogenation of Cumene over Fe2O3-Cr2O3-K2CO3 Catalyst Hiroshi MIURA*, Satoshi TAKAHASHI, Yohji MIZUSHIMA, Kazuo SUGIYAMA, and Tsuneo MATSUDA Department of Applied Chemistry, Saitama University, Shimo-okubo, Urawa-shi 338 (Received July 29, 1988) The rate of dehydrogenation of cumene over Fe2O3-Cr2O3-K2CO3 catalyst to derive α-methylstyrene has been studied. The reaction rate has been interpreted on the basis of a reaction model taking account of competitive adsorption of cumene, α-methylstyrene, styrene and CO2. The kinetic parameters were compared with those of dehydrogenation of ethylbenzene. The dehydrogenation of cumene proceeded more rapidly than that of ethylbenzene because the effect of product retardation was less serious. operated at ambient pressure. Kinetic parameters 1. Introduction were obtained by means of differential reactor While detailed studies have been made on the technique, keeping the cumene conversion less dehydrogenation of ethylbenzene1)-5), there have than 10% (in most cases less than 5%). Such been only few papers on the dehydrogenation of experiments were carried out at a temperature of alkylbenzenes other than ethylbenzene.5),7),8) 535℃, with catalyst weighing 0.5g, molar ratio The decomposition of cumene has frequently of cumene/H2O=1/12 and contact time of 10- been tried in studying the acid-base properties of 30g・h/mol. The rate equation thus obtained was oxide catalysts, since cumene is decomposed to verified along with the data obtained using the benzene and propylene over Bronsted acid catalysts9), integral reactor, in which case cumene conversion whereas it is dehydrogenated over base catalysts. -

Department of Environmental Protection
E. Analytical Requirements The following table specifies the analytical requirements for soil and water samples collected during the site assessment to determine whether a storage tank site may be closed pursuant to this guidance document or is subject to the corrective action process regulations. Other recognized methods may be used if approved by the appropriate DEP regional office. ANALYTICAL METHOD PRODUCT PARAMETERS TO BE (reported on a PARAMETERS TO BE ANALYTICAL METHOD STORED TESTED IN SOIL dry weight basis) TESTED IN WATER Leaded Gasoline, Benzene EPA Method 5035/8021B or Benzene EPA Method 5030B/8021B, Aviation Gasoline, Toluene 5035/8260B Toluene 5030B/8260B or 524.2 and Jet Fuel Ethyl Benzene Ethyl Benzene Xylenes (total) Xylenes (total) Cumene (Isopropylbenzene) Cumene (Isopropylbenzene) Naphthalene Naphthalene Trimethyl benzene, 1,2,4- Trimethyl benzene, 1,2,4- (Trimethyl benzene, 1,3,4-) (Trimethyl benzene, 1,3,4-) Trimethyl benzene, 1,3,5- Trimethyl benzene, 1,3,5- Dichloroethane, 1,2- Dichloroethane, 1,2- Dibromoethane, 1,2- Dibromoethane, 1,2- EPA Method 8011 or 504.1 Lead (total) EPA Method 6010B or 7420 Lead (dissolved) EPA Method 6020 or 7421 Unleaded Benzene EPA Method 5035/8260B Benzene EPA Method 5030B/8260B Gasoline Toluene Toluene or 524.2 Ethyl Benzene Ethyl Benzene Xylenes (total) Xylenes (total) Cumene (Isopropylbenzene) Cumene (Isopropylbenzene) Methyl tert-Butyl Ether Methyl tert-Butyl Ether Naphthalene Naphthalene Trimethyl benzene, 1,2,4- Trimethyl benzene, 1,2,4- (Trimethyl benzene, 1,3,4-) (Trimethyl benzene, 1,3,4-) Trimethyl benzene, 1,3,5- Trimethyl benzene, 1,3,5- Kerosene, Benzene EPA Method 5035/8260B Benzene EPA Method 5030B/8260B Fuel Oil No. -

Material Safety Data Sheet
MATERIAL SAFETY DATA SHEET 1. CHEMICAL PRODUCT AND COMPANY INFORMATION Product Name: SUNOCO SUPREME Manufacturer Information: Sunoco, Inc. (R&M) 1735 Market Street LL Philadelphia, Pennsylvania, 19103-7583 Product Use: Racing fuel Emergency Phone Numbers: Chemtrec (800) 424-9300 Sunoco Inc. (800) 964-8861 Information: Product Safety Information (610) 859-1120 2. COMPOSITION/INFORMATION ON INGREDIENTS Component CAS No. Amount (Vol%) LIGHT PETROLEUM DISTILLATE 8006-61-9 99.9 - 99.9 TOLUENE 108-88-3 0 - 30 XYLENE 1330-20-7 0 - 25 CYCLOHEXANE 110-82-7 0 - 9 ETHYL BENZENE 100-41-4 0 - 5 N-HEXANE 110-54-3 0 - 5 NAPHTHALENE 91-20-3 0 - 5 1,2,4-TRIMETHYLBENZENE 95-63-6 0 - 5 BENZENE 71-43-2 0.1 - 4.9 CUMENE 98-82-8 0 - 1 TETRAETHYL LEAD 78-00-2 0.092 - 0.12 EXPOSURE GUIDELINES (SEE SECTION 15 FOR ADDITIONAL EXPOSURE LIMITS) CAS No. Governing Body Exposure Limits BENZENE 71-43-2 ACGIH STEL 2.5 ppm BENZENE 71-43-2 OSHA STEL 5 ppm BENZENE 71-43-2 ACGIH TWA 0.5 ppm BENZENE 71-43-2 OSHA TWA 1 ppm CUMENE 98-82-8 ACGIH TWA 50 ppm CUMENE 98-82-8 OSHA TWA 50 ppm CYCLOHEXANE 110-82-7 ACGIH TWA 100 ppm CYCLOHEXANE 110-82-7 OSHA TWA 300 ppm ETHYL BENZENE 100-41-4 ACGIH STEL 125 ppm ETHYL BENZENE 100-41-4 ACGIH TWA 100 ppm ETHYL BENZENE 100-41-4 OSHA TWA 100 ppm N-HEXANE 110-54-3 ACGIH TWA 50 ppm N-HEXANE 110-54-3 OSHA TWA 500 ppm NAPHTHALENE 91-20-3 ACGIH STEL 15 ppm NAPHTHALENE 91-20-3 ACGIH TWA 10 ppm NAPHTHALENE 91-20-3 OSHA TWA 10 ppm TOLUENE 108-88-3 NIOSH STEL 150 ppm TOLUENE 108-88-3 ACGIH TWA 50 ppm TOLUENE 108-88-3 OSHA TWA 200 ppm XYLENE 1330-20-7 ACGIH STEL 150 ppm XYLENE 1330-20-7 ACGIH TWA 100 ppm XYLENE 1330-20-7 OSHA TWA 100 ppm LIGHT PETROLEUM 8006-61-9 ACGIH STEL 500 ppm DISTILLATE LIGHT PETROLEUM 8006-61-9 ACGIH TWA 300 ppm DISTILLATE TETRAETHYL LEAD 78-00-2 ACGIH TWA 0.1 mg/m3 TETRAETHYL LEAD 78-00-2 OSHA TWA 0.075 mg/m3 3. -

EPA Region 3 RCRA Corrective Action Environmental Indicator For
DOCUMENTATION OF ENVIRONMENTAL INDICATOR DETERMINATION Interim Final 2/5/99 RCRA Corrective Action Environmental Indicator (EI) RCRIS code (CA725) Current Human Exposures Under Control Facility Name: Kinder Morgan Liquids Terminals (Formerly Unitank Terminals Service) Facility Address: Allegheny Avenue and Delaware River Facility EPA ID #: PAD087098653 1. Has all available relevant/significant information on known and reasonably suspected releases to soil, groundwater, surface water/sediments, and air, subject to RCRA Corrective Action (e.g., from Solid Waste Management Units (SWMU), Regulated Units (RU), and Areas of Concern (AOC)), been considered in this EI determination? X If yes - check here and continue with #2 below. _____ If no - re-evaluate existing data, or _____ if data are not available skip to #6 and enter“IN” (more information needed) status code. BACKGROUND Definition of Environmental Indicators (for the RCRA Corrective Action) Environmental Indicators (EI) are measures being used by the RCRA Corrective Action program to go beyond programmatic activity measures (e.g., reports received and approved, etc.) to track changes in the quality of the environment. The two EI developed to-date indicate the quality of the environment in relation to current human exposures to contamination and the migration of contaminated groundwater. An EI for non-human (ecological) receptors is intended to be developed in the future. Definition of “Current Human Exposures Under Control” EI A positive “Current Human Exposures Under Control” EI determination (“YE” status code) indicates that there are no “unacceptable” human exposures to “contamination” (i.e., contaminants in concentrations in excess of appropriate risk-based levels) that can be reasonably expected under current land- and groundwater-use conditions (for all “contamination” subject to RCRA corrective action at or from the identified facility (i.e., site-wide)). -

Cumene Contamination in Groundwater: Observed Concentrations, Evaluation of Remediation by Sulfate Enhanced Bioremediation (SEB), and Public Health Issues
International Journal of Environmental Research and Public Health Review Cumene Contamination in Groundwater: Observed Concentrations, Evaluation of Remediation by Sulfate Enhanced Bioremediation (SEB), and Public Health Issues John P. Herman 1, Lauren Redfern 2 , Christopher Teaf 3 , Douglas Covert 4, Peter R. Michael 1 and Thomas M. Missimer 1,* 1 Emergent Technologies Institute, U. A. Whitaker College of Engineering, Florida Gulf Coast University, Fort Myers, FL 33901, USA; [email protected] (J.P.H.); [email protected] (P.R.M.) 2 U. A. Whitaker College of Engineering, Florida Gulf Coast College, Fort Myers, FL 33965, USA; [email protected] 3 Institute for Science & Public Affairs, Florida State University, Tallahassee, FL 32306, USA; [email protected] 4 Hazardous Substance and Waste Management Research, Tallahassee, FL 32309, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-239-745-4538 Received: 13 October 2020; Accepted: 10 November 2020; Published: 12 November 2020 Abstract: Isopropylbenzene (cumene) is commonly encountered in groundwater at petroleum release sites due to its natural occurrence in crude oil and historical use as a fuel additive. The cumene concentrations detected at these sites often exceed regulatory guidelines or standards for states with stringent groundwater regulations. Recent laboratory analytical data collected at historical petroleum underground storage tank (UST) release sites have revealed that cumene persists at concentrations exceeding the default cleanup criterion, while other common petroleum constituents are below detection limits or low enough to allow natural attenuation as a remediation strategy. This effectively makes cumene the driver for active remediation at some sites. An insignificant amount of research has been conducted for the in-situ remediation of cumene.