210 Nielsen Layout 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gyrfalcon Falco Rusticolus
Gyrfalcon Falco rusticolus Rob Florkiewicz surveys, this area was included. Eight eyries are known from this Characteristics and Range The northern-dwelling Gyrfalcon is part of the province; however, while up to 7 of these eyries have the largest falcon in the world. It breeds mostly along the Arctic been deemed occupied in a single year, no more than 3 have been coasts of North America, Europe and Asia (Booms et al. 2008). productive at the same time. Based on these data and other Over its range, its colour varies from white through silver-grey to sightings, the British Columbia Wildlife Branch estimates the almost black; silver-grey is the most common morph in British breeding population in the province to be fewer than 20 pairs Columbia. It nests on cliff ledges at sites that are often used for (Chutter 2008). decades and where considerable amounts of guano can accumulate. Ptarmigan provide the Gyrfalcon's main prey in In British Columbia, the Gyrfalcon nests on cliff ledges on British Columbia and productivity appears dependent on mountains in alpine areas, usually adjacent to rivers or lakes. ptarmigan numbers. Large size and hunting prowess make the Occasionally, it nests on cliffs of river banks and in abandoned Gyrfalcon a popular bird with falconers, who breed and train Golden Eagle nests. them to hunt waterfowl and other game birds. Conservation and Recommendations Whilst the Gyrfalcon is Distribution, Abundance, and Habitat Most Gyrfalcons breed designated as Not at Risk nationally by COSEWIC, it is Blue-listed along the Arctic coast; however, a few breed in the northwest in British Columbia due to its small known breeding population portion of the Northern Boreal Mountains Ecoprovince of British (British Columbia Ministry of Environment 2014). -

Gyrfalcon Diet in Central West Greenland During the Nesting Period
The Condor 105:528±537 q The Cooper Ornithological Society 2003 GYRFALCON DIET IN CENTRAL WEST GREENLAND DURING THE NESTING PERIOD TRAVIS L. BOOMS1,3 AND MARK R. FULLER2 1Boise State University Raptor Research Center, 1910 University Drive, Boise, ID 83725 2USGS Forest and Rangeland Ecosystem Science Center, 970 Lusk St., Boise, ID 83706 Abstract. We studied food habits of Gyrfalcons (Falco rusticolus) nesting in central west Greenland in 2000 and 2001 using three sources of data: time-lapse video (3 nests), prey remains (22 nests), and regurgitated pellets (19 nests). These sources provided different information describing the diet during the nesting period. Gyrfalcons relied heavily on Rock Ptarmigan (Lagopus mutus) and arctic hares (Lepus arcticus). Combined, these species con- tributed 79±91% of the total diet, depending on the data used. Passerines were the third most important group. Prey less common in the diet included waterfowl, arctic fox pups (Alopex lagopus), shorebirds, gulls, alcids, and falcons. All Rock Ptarmigan were adults, and all but one arctic hare were young of the year. Most passerines were ¯edglings. We observed two diet shifts, ®rst from a preponderance of ptarmigan to hares in mid-June, and second to passerines in late June. The video-monitored Gyrfalcons consumed 94±110 kg of food per nest during the nestling period, higher than previously estimated. Using a combi- nation of video, prey remains, and pellets was important to accurately document Gyrfalcon diet, and we strongly recommend using time-lapse video in future diet studies to identify biases in prey remains and pellet data. Key words: camera, diet, Falco rusticolus, food habits, Greenland, Gyrfalcon, time-lapse video. -

Natural History of the Gyrfalcon in the Central Canadian Arctic K.G
ARCTIC VOL. 41, NO. 1 (MARCH 1988) P. 31-38 Natural History of the Gyrfalcon in the Central Canadian Arctic K.G. POOLE' and R.G. BROMLEY2 (Received 24 March 1987; accepted in revised form 24 September 1987) ABSTRACT. A population of breeding gyrfalcons was studied from 1982to 1986 on 2000a km2 area in thecentral Arctic of the NorthwestTerritories. Each year 14-18 territories were occupied. The meanintemest distance was 10.6 km, giving oneof the highest recorded densitiesfor the species. There was a tendency for regularity in spacing of territories. Most (85%) nests were in abandoned stick nests of common ravens or golden eagles. Rough-legged hawk nests were not used by gyrfalcons, despite numerous available.date Mean of initiation of laying was 8 May. Meansize of clutch was 3.80 and of brood was 2.53, and mean productivity was 1SO fledged young. A reduction of 48% from estimated numberof eggs laid to number of fledglings was determined. Reproductive success declined with increased severity of spring weather, notablydays increased and amount of precipitation. Key words: gyrfalcon (Falco rusticolus),natural history, reproductive ecology, central Arctic RÉSUMÉ. Qtre 1982 et 1986, on a étudié une populationde gerfauts en reproduction dans une zone de 2000 km2dans la région centrale arctiquedes Territoires du Nord-Ouest. Quatorze des 18 aires étaient occupées chaque année. La distance entremoyenne les nids étaitde 10,6km, soit la plus grande densité relevée pour cetteespèce. Les aires avaient tendanceB être espacéesrégulitrement. La plupart des nids(85 p. cent) étaient situésdans des nids de brindilles occupés précédemment pardes corbeaux communsou des aigles dorés. -

Nesting Behavior of Female White-Tailed Ptarmigan in Colorado
SHORT COMMUNICATIONS 215 Condor, 81:215-217 0 The Cooper Ornithological Society 1070 NESTING BEHAVIOR OF FEMALE settling on the clutches. By lifting the hens off their WHITE-TAILED PTARMIGAN nests, we learned that eggs were laid almost immedi- ately after settling. IN COLORADO After eggs were laid, the hens remained relatively inactive until they prepared to depart from the nest. Observations of six hens in 1975 indicated that they KENNETH M. GIESEN remained on the nest for longer periods as the clutch AND approached completion. One hen depositing her sec- ond egg remained on her nest for 44 min, whereas CLAIT E. BRAUN another, depositing the fifth egg of a six-egg clutch, remained on the nest more than 280 min. Three hens remained on their nests 84 to 153 min when laying Few detailed observations on behavior of nesting their second or third eggs. Spruce Grouse also show grouse have been reported. Notable exceptions are this pattern of nest attentiveness (McCourt et al. those of SchladweiIer (1968) and Maxson (1977) 1973). who studied feeding behavior and activity patterns of Before departing from the nest, the hen began to Ruffed Grouse (Bonasa umbellus) in Minnesota, and peck at vegetation and place it at the rim of the McCourt et al. ( 1973) who documented nest atten- nest, or throw it over her back. This behavior lasted tiveness of Spruce Grouse (Canachites canudensis) in 34, 40 and 64 min for three hens. Vegetation was southwestern Alberta. White-tailed Ptarmigan (Lugo- deposited on the nest at the rate of 20 pieces per pus Zeucurus) have been intensively studied in Colo- minute. -
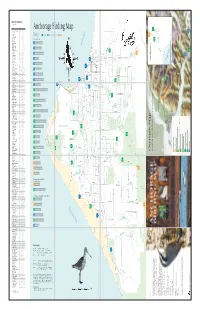
Anchorage Birding Map ❏ Common Redpoll* C C C C ❄ ❏ Hoary Redpoll R ❄ ❏ Pine Siskin* U U U U ❄ Additional References: Anchorage Audubon Society
BIRDS OF ANCHORAGE (Knik River to Portage) SPECIES SP S F W ❏ Greater White-fronted Goose U R ❏ Snow Goose U ❏ Cackling Goose R ? ❏ Canada Goose* C C C ❄ ❏ Trumpeter Swan* U r U ❏ Tundra Swan C U ❏ Gadwall* U R U ❄ ❏ Eurasian Wigeon R ❏ American Wigeon* C C C ❄ ❏ Mallard* C C C C ❄ ❏ Blue-winged Teal r r ❏ Northern Shoveler* C C C ❏ Northern Pintail* C C C r ❄ ❏ Green-winged Teal* C C C ❄ ❏ Canvasback* U U U ❏ Redhead U R R ❄ ❏ Ring-necked Duck* U U U ❄ ❏ Greater Scaup* C C C ❄ ❏ Lesser Scaup* U U U ❄ ❏ Harlequin Duck* R R R ❄ ❏ Surf Scoter R R ❏ White-winged Scoter R U ❏ Black Scoter R ❏ Long-tailed Duck* R R ❏ Bufflehead U U ❄ ❏ Common Goldeneye* C U C U ❄ ❏ Barrow’s Goldeneye* U U U U ❄ ❏ Common Merganser* c R U U ❄ ❏ Red-breasted Merganser u R ❄ ❏ Spruce Grouse* U U U U ❄ ❏ Willow Ptarmigan* C U U c ❄ ❏ Rock Ptarmigan* R R R R ❄ ❏ White-tailed Ptarmigan* R R R R ❄ ❏ Red-throated Loon* R R R ❏ Pacific Loon* U U U ❏ Common Loon* U R U ❏ Horned Grebe* U U C ❏ Red-necked Grebe* C C C ❏ Great Blue Heron r r ❄ ❏ Osprey* R r R ❏ Bald Eagle* C U U U ❄ ❏ Northern Harrier* C U U ❏ Sharp-shinned Hawk* U U U R ❄ ❏ Northern Goshawk* U U U R ❄ ❏ Red-tailed Hawk* U R U ❏ Rough-legged Hawk U R ❏ Golden Eagle* U R U ❄ ❏ American Kestrel* R R ❏ Merlin* U U U R ❄ ❏ Gyrfalcon* R ❄ ❏ Peregrine Falcon R R ❄ ❏ Sandhill Crane* C u U ❏ Black-bellied Plover R R ❏ American Golden-Plover r r ❏ Pacific Golden-Plover r r ❏ Semipalmated Plover* C C C ❏ Killdeer* R R R ❏ Spotted Sandpiper* C C C ❏ Solitary Sandpiper* u U U ❏ Wandering Tattler* u R R ❏ Greater Yellowlegs* -

Europe's Huntable Birds a Review of Status and Conservation Priorities
FACE - EUROPEAN FEDERATIONEurope’s FOR Huntable HUNTING Birds A Review AND CONSERVATIONof Status and Conservation Priorities Europe’s Huntable Birds A Review of Status and Conservation Priorities December 2020 1 European Federation for Hunting and Conservation (FACE) Established in 1977, FACE represents the interests of Europe’s 7 million hunters, as an international non-profit-making non-governmental organisation. Its members are comprised of the national hunters’ associations from 37 European countries including the EU-27. FACE upholds the principle of sustainable use and in this regard its members have a deep interest in the conservation and improvement of the quality of the European environment. See: www.face.eu Reference Sibille S., Griffin, C. and Scallan, D. (2020) Europe’s Huntable Birds: A Review of Status and Conservation Priorities. European Federation for Hunting and Conservation (FACE). https://www.face.eu/ 2 Europe’s Huntable Birds A Review of Status and Conservation Priorities Executive summary Context Non-Annex species show the highest proportion of ‘secure’ status and the lowest of ‘threatened’ status. Taking all wild birds into account, The EU State of Nature report (2020) provides results of the national the situation has deteriorated from the 2008-2012 to the 2013-2018 reporting under the Birds and Habitats directives (2013 to 2018), and a assessments. wider assessment of Europe’s biodiversity. For FACE, the findings are of key importance as they provide a timely health check on the status of In the State of Nature report (2020), ‘agriculture’ is the most frequently huntable birds listed in Annex II of the Birds Directive. -

North American Game Birds Or Animals
North American Game Birds & Game Animals LARGE GAME Bear: Black Bear, Brown Bear, Grizzly Bear, Polar Bear Goat: bezoar goat, ibex, mountain goat, Rocky Mountain goat Bison, Wood Bison Moose, including Shiras Moose Caribou: Barren Ground Caribou, Dolphin Caribou, Union Caribou, Muskox Woodland Caribou Pronghorn Mountain Lion Sheep: Barbary Sheep, Bighorn Deer: Axis Deer, Black-tailed Deer, Sheep, California Bighorn Sheep, Chital, Columbian Black-tailed Deer, Dall’s Sheep, Desert Bighorn Mule Deer, White-tailed Deer Sheep, Lanai Mouflon Sheep, Nelson Bighorn Sheep, Rocky Elk: Rocky Mountain Elk, Tule Elk Mountain Bighorn Sheep, Stone Sheep, Thinhorn Mountain Sheep Gemsbok SMALL GAME Armadillo Marmot, including Alaska marmot, groundhog, hoary marmot, Badger woodchuck Beaver Marten, including American marten and pine marten Bobcat Mink North American Civet Cat/Ring- tailed Cat, Spotted Skunk Mole Coyote Mouse Ferret, feral ferret Muskrat Fisher Nutria Fox: arctic fox, gray fox, red fox, swift Opossum fox Pig: feral swine, javelina, wild boar, Lynx wild hogs, wild pigs Pika Skunk, including Striped Skunk Porcupine and Spotted Skunk Prairie Dog: Black-tailed Prairie Squirrel: Abert’s Squirrel, Black Dogs, Gunnison’s Prairie Dogs, Squirrel, Columbian Ground White-tailed Prairie Dogs Squirrel, Gray Squirrel, Flying Squirrel, Fox Squirrel, Ground Rabbit & Hare: Arctic Hare, Black- Squirrel, Pine Squirrel, Red Squirrel, tailed Jackrabbit, Cottontail Rabbit, Richardson’s Ground Squirrel, Tree Belgian Hare, European -

Revision of Molt and Plumage
The Auk 124(2):ART–XXX, 2007 © The American Ornithologists’ Union, 2007. Printed in USA. REVISION OF MOLT AND PLUMAGE TERMINOLOGY IN PTARMIGAN (PHASIANIDAE: LAGOPUS SPP.) BASED ON EVOLUTIONARY CONSIDERATIONS Peter Pyle1 The Institute for Bird Populations, P.O. Box 1346, Point Reyes Station, California 94956, USA Abstract.—By examining specimens of ptarmigan (Phasianidae: Lagopus spp.), I quantifi ed three discrete periods of molt and three plumages for each sex, confi rming the presence of a defi nitive presupplemental molt. A spring contour molt was signifi cantly later and more extensive in females than in males, a summer contour molt was signifi cantly earlier and more extensive in males than in females, and complete summer–fall wing and contour molts were statistically similar in timing between the sexes. Completeness of feather replacement, similarities between the sexes, and comparison of molts with those of related taxa indicate that the white winter plumage of ptarmigan should be considered the basic plumage, with shi s in hormonal and endocrinological cycles explaining diff erences in plumage coloration compared with those of other phasianids. Assignment of prealternate and pre- supplemental molts in ptarmigan necessitates the examination of molt evolution in Galloanseres. Using comparisons with Anserinae and Anatinae, I considered a novel interpretation: that molts in ptarmigan have evolved separately within each sex, and that the presupplemental and prealternate molts show sex-specifi c sequences within the defi nitive molt cycle. Received 13 June 2005, accepted 7 April 2006. Key words: evolution, Lagopus, molt, nomenclature, plumage, ptarmigan. Revision of Molt and Plumage Terminology in Ptarmigan (Phasianidae: Lagopus spp.) Based on Evolutionary Considerations Rese.—By examining specimens of ptarmigan (Phasianidae: Lagopus spp.), I quantifi ed three discrete periods of molt and three plumages for each sex, confi rming the presence of a defi nitive presupplemental molt. -

A Bird the Color of Winter in Above & Beyond, 2018
A Bird the Colour of Winter From delicacy to cultural icon By Michael Engelhard Nunavut’s territorial bird confounds southerners and even some residents. English speakers routinely pronounce the p, which really is silent. And therein lies a tale. Ptarmigan comes from the Gaelic, tàrmachan , for “grumbler” or “croaker,” a reminder of the ptarmigan’s grouchy voice. But in 1684, the Scottish naturalist Sir Robert Sibbald, added the unpronounced p, falsely suggesting a Greek word origin (as in ptero- , “feather” or “wing”). Perhaps this learned northern gent simply slipped or else tried to elevate the monkish, pedestrian bird. Overwintering in the Arctic, as do redpolls, ravens, and snowy ptarmigan, in contrast with its cousins, is not circumpolar. Its owls — only eleven tribes of Aves live there year-round — ptarmigans realms are spiny heights, alpine ridges and meadows of southern by the hundreds come together for the dark season. Three domestic Alaska, the Pacific Northwest’s coastal ranges, and the Rockies. kinds occupy different niches: willow ptarmigan (also “willow Quick-change artists, all three species switch from solid umber, grouse,” or “red grouse,” in Britain) prefer boreal forest and wetlands; chestnut, and black-barred gold to mottled to all-cream plumage the most northerly of terrestrial birds, rock ptarmigan (formerly and back during a single year. The sun’s shifting arc — amounts known as “snow chicken” or “white pheasant”) feel at home in of daylight or “photoperiod” — triggers these seasonal makeovers. drier foothills and uplands. The less well-known white-tailed Unlike geese and ducks, ptarmigan never become flightless because they replace feathers sequentially. -

Movement, Survival, and Nest Monitoring of Rock Ptarmigan in Game Management Unit 13B, 2013–2017
Final Wildlife Research Report ADF&G/DWC/WRR-2018-1 Movement, Survival, and Nest Monitoring of Rock Ptarmigan in Game Management Unit 13B, 2013–2017 Richard A. Merizon, John P. Skinner, and Miles O. Spathelf ©2014 ADF&G. Photo by Dale Rabe. 2018 Alaska Department of Fish and Game Division of Wildlife Conservation Final Wildlife Research Report ADF&G/DWC/WRR-2018-1 Movement, Survival, and Nest Monitoring of Rock Ptarmigan in Game Management Unit 13B, 2013–2017 Richard A. Merizon Alaska Department of Fish and Game 1800 Glenn Highway, Suite 2 Palmer, AK 99645 [email protected] (907) 746-6333 John P. Skinner Alaska Department of Fish and Game 333 Raspberry Road Anchorage, AK 99518 [email protected] (907) 267-2283 Miles O. Spathelf Alaska Department of Fish and Game 333 Raspberry Road Anchorage, AK 99518 [email protected] (907) 267-2463 ©2018 Alaska Department of Fish and Game Alaska Department of Fish and Game Division of Wildlife Conservation PO Box 115526 Juneau, AK 99811-5526 This project was funded in part by Federal Aid in Wildlife Restoration Grant F12AF00050. Final wildlife research reports are final reports detailing the objectives, methods, data collected and findings of a particular research project undertaken by ADF&G Division of Wildlife Conservation staff and partners. They are written to provide broad access to information obtained through the project. While these are final reports, further data analysis may result in future adjustments to the conclusions. Please contact the author(s) prior to citing material in these reports. These reports are professionally reviewed by research staff in the Division of Wildlife Conservation. -

WBRC Review List 2020
Official Washington State Checklist of Birds (updated by Washington Bird Records Committee, Nov 2020) __Fulvous Whistling-Duck (1905) __White-throated Swift __Long-tailed Jaeger __Nazca Booby __Tropical Kingbird __Dusky Thrush (s) __Tricolored Blackbird __Emperor Goose __Ruby-throated Hummingbird __Common Murre __Blue-footed Booby __Western Kingbird __Redwing __Brown-headed Cowbird __Snow Goose __Black-chinned Hummingbird __Thick-billed Murre __Brown Booby __Eastern Kingbird __American Robin __Rusty Blackbird __Ross’s Goose __Anna’s Hummingbird __Pigeon Guillemot __Red-footed Booby __Scissor-tailed Flycatcher __Varied Thrush __Brewer’s Blackbird __Gr. White-fronted Goose __Costa’s Hummingbird __Long-billed Murrelet __Brandt’s Cormorant __Fork-tailed Flycatcher __Gray Catbird __Common Grackle __Taiga Bean-Goose __Calliope Hummingbird __Marbled Murrelet __Pelagic Cormorant __Olive-sided Flycatcher __Brown Thrasher __Great-tailed Grackle __Brant __Rufous Hummingbird __Kittlitz’s Murrelet __Double-crested Cormorant __Greater Pewee (s) __Sage Thrasher __Ovenbird __Cackling Goose __Allen’s Hummingbird (1894) __Scripps’s Murrelet __American White Pelican __Western Wood-Pewee __Northern Mockingbird __Northern Waterthrush __Canada Goose __Broad-tailed Hummingbird __Guadalupe Murrelet __Brown Pelican __Eastern Wood-Pewee __European Starling (I) __Golden-winged Warbler __Trumpeter Swan __Broad-billed Hummingbird __Ancient Murrelet __American Bittern __Yellow-bellied Flycatcher __Bohemian Waxwing __Blue-winged Warbler __Tundra Swan __Virginia Rail -

Alpha Codes for 2168 Bird Species (And 113 Non-Species Taxa) in Accordance with the 62Nd AOU Supplement (2021), Sorted Taxonomically
Four-letter (English Name) and Six-letter (Scientific Name) Alpha Codes for 2168 Bird Species (and 113 Non-Species Taxa) in accordance with the 62nd AOU Supplement (2021), sorted taxonomically Prepared by Peter Pyle and David F. DeSante The Institute for Bird Populations www.birdpop.org ENGLISH NAME 4-LETTER CODE SCIENTIFIC NAME 6-LETTER CODE Highland Tinamou HITI Nothocercus bonapartei NOTBON Great Tinamou GRTI Tinamus major TINMAJ Little Tinamou LITI Crypturellus soui CRYSOU Thicket Tinamou THTI Crypturellus cinnamomeus CRYCIN Slaty-breasted Tinamou SBTI Crypturellus boucardi CRYBOU Choco Tinamou CHTI Crypturellus kerriae CRYKER White-faced Whistling-Duck WFWD Dendrocygna viduata DENVID Black-bellied Whistling-Duck BBWD Dendrocygna autumnalis DENAUT West Indian Whistling-Duck WIWD Dendrocygna arborea DENARB Fulvous Whistling-Duck FUWD Dendrocygna bicolor DENBIC Emperor Goose EMGO Anser canagicus ANSCAN Snow Goose SNGO Anser caerulescens ANSCAE + Lesser Snow Goose White-morph LSGW Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Intermediate-morph LSGI Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Blue-morph LSGB Anser caerulescens caerulescens ANSCCA + Greater Snow Goose White-morph GSGW Anser caerulescens atlantica ANSCAT + Greater Snow Goose Intermediate-morph GSGI Anser caerulescens atlantica ANSCAT + Greater Snow Goose Blue-morph GSGB Anser caerulescens atlantica ANSCAT + Snow X Ross's Goose Hybrid SRGH Anser caerulescens x rossii ANSCAR + Snow/Ross's Goose SRGO Anser caerulescens/rossii ANSCRO Ross's Goose