Epalzeorhynchos Bicolor) Ecological Risk Screening Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Rainbow Sharkminnow (Epalzeorhynchos Frenatum) ERSS
Rainbow Sharkminnow (Epalzeorhynchos frenatum) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, June 2012 Revised, November 2018 Web Version, 2/1/2019 Photo: Drachenschwertträger36/Wikimedia. Licensed under Creative Commons BY-SA 2.0 Germany. Available: https://commons.wikimedia.org/wiki/File:Z%C3%BCgelfransenlipper.jpg. (November 8, 2018). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2018): “Asia: Mekong, Chao Phraya and Xe Bangfai basins [Kottelat 1998] and Maeklong basin [Yang and Winterbottom 1998].” 1 “[In Cambodia:] Occurs in the Mekong River [Kottelat 1998]. Known from Tonlé Sap and Phnom Penh [Kottelat 1985].” “[In Laos:] Occurs in the Mekong and the lower Xe Bangfai [Kottelat 1998]. Found in Ban Hang Khone, a village on an island in the middle of the mainstream Mekong River just below the Great Khone Waterfalls in Khong District, Champasak Province [Baird 1998].” “[In Thailand:] Occurs in the Maeklong [Vidthayanon et al. 1997], Mekong and Chao Phraya basins [Vidthayanon et al. 1997; Kottelat 1998]. Known from Chiang Mai and the Tachin (Samut Sakhon) River [Suvatti 1981]; also from Phra Nakhon Si Ayutthaya, Nakhon Sawan, Ubon Ratchathani, Kanchanaburi, Nakhon Ratchasima, Phrae and Phitsanulok [Monkolprasit et al. 1997].” From Vidthayanon (2012): “It has also been reported from Viet Nam.” Status in the United States Tuckett et al. (2017) caught 1 individual of Epalzeorhynchos frenatum in the wild in Florida but within 500m of an aquaculture facility. According to Chapman et al. (1994), Epalzeorhynchos frenatum (listed as Labeo frenatus) was imported to the United States for the ornamental trade in 1992. -

Epalzeorhynchos Munense ERSS
Epalzeorhynchos munense (a fish, no common name) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, June 2012 Revised, November 2018 Web Version, 2/1/2019 Photo: W. J. Rainboth/FAO. Licensed under Creative Commons BY-NC 3.0 Unported. Available: http://www.fishbase.org/photos/PicturesSummary.php?StartRow=0&ID=27163&what=species& TotRec=2. (November 9, 2018). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2018a): “Asia: Mekong [Cambodia, Thailand, Laos] and Chao Phraya [Thailand] basins [Kottelat 2001] and Maeklong basin [Thailand] [Vidthayanon et al. 1997].” “[In Laos:] Known from the middle Xe Bangfai, a tributary of the Mekong basin [Kottelat 1998].” 1 Status in the United States No records of Epalzeorhynchos munense in the wild or in trade in the United States were found. Means of Introductions in the United States No records of Epalzeorhynchos munense in the wild in the United States were found. Remarks Information searches were conducted using the valid name Epalzeorhynchos munense and the synonym Labeo munensis. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing According to Fricke et al. (2018), Epalzeorhynchos munense is the valid name for this species. It was originally described as Labeo munensis. From Froese and Pauly (2018b): “Animalia (Kingdom) > Chordata (Phylum) > Vertebrata (Subphylum) > Gnathostomata (Superclass) > […] Actinopterygii (Class) > Cypriniformes (Order) > Cyprinidae (Family) > Epalzeorhynchos munense (Species)” Size, Weight, and Age Range From Froese and Pauly (2018a): “Max length : 9.3 cm SL male/unsexed; [Kottelat 1998]” Environment From Froese and Pauly (2018a): “Freshwater; benthopelagic.” Climate/Range From Froese and Pauly (2018a): “Tropical” 2 Distribution Outside the United States Native From Froese and Pauly (2018a): “Asia: Mekong [Cambodia, Thailand, Laos] and Chao Phraya [Thailand] basins [Kottelat 2001] and Maeklong basin [Thailand] [Vidthayanon et al. -

Induction of Maturation and Ovulation of Red Fin Shark Fish
International Journal of Fisheries and Aquatic Studies 2017; 5(4): 418-424 E-ISSN: 2347-5129 P-ISSN: 2394-0506 (ICV-Poland) Impact Value: 5.62 Induction of maturation and ovulation of Red Fin (GIF) Impact Factor: 0.549 IJFAS 2017; 5(4): 418-424 Shark fish Epalzeorhynchos frenatus in non-spawning © 2017 IJFAS www.fisheriesjournal.com season Received: 03-05-2017 Accepted: 04-06-2017 Muhammad Faiz Islami, Agus Oman Sudrajat and Odang Carman Muhammad Faiz Islami Department of Aquaculture, Bogor Agricultural University, Abstract Bogor, Indonesia, Jl. Agatis There are constraints on the production of red fin shark Epalzeorhynchos frenatus due to the lack of Kampus Dramaga, Bogor, matured broodstocks in Non-spawning season and has a dependence on hormone induction during Indonesia spawning process, because it can’t spawn naturally in cultivation environments. Gonad maturation can be stimulated by the addition of hormones and potential plant-based materials to feed. Thus, the present Agus Oman Sudrajat study attempted alternative hormonal combination to induce both ovulation and spawning in order to Department of Aquaculture, provide farmers with alternative choices for reproduction. This research was divided into two sub- Bogor Agricultural University, research (maturation and ovulation) in one main title. The first section of the study dealt with Bogor, Indonesia, Jl. Agatis maturational induction and it used Completely Randomized Design with 5 treatments. Ten broodstocks Kampus Dramaga, Bogor, were used in each treatment which was as follows: Oodev (0.25 and 0.5 mL. kg-1 fish) addition to feed, Indonesia turmeric powder (250 and 500 mg 100 g-1 feed), and commercial feed (at least 30 % protein) as the control. -

Comparison of Evolutionary Rates in the Mitochondrial DNA Cytochrome B Gene and Control Region and Their Implications for Phylog
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Institute of Hydrobiology, Chinese Academy Of Sciences Molecular Phylogenetics and Evolution 39 (2006) 347–357 www.elsevier.com/locate/ympev Comparison of evolutionary rates in the mitochondrial DNA cytochrome b gene and control region and their implications for phylogeny of the Cobitoidea (Teleostei: Cypriniformes) Qiongying Tang a,b, Huanzhang Liu a,¤, Richard Mayden c, Bangxi Xiong b a Institute of Hydrobiology, Chinese Academy of Sciences, Hubei, Wuhan 430072, PR China b College of Fishery, Huazhong Agricultural University, Hubei, Wuhan 430070, PR China c Department of Biology, Saint Louis University, 3507 Laclede Ave., St. Louis, MO 63103-2010, USA Received 6 July 2005; revised 15 August 2005; accepted 18 August 2005 Available online 4 October 2005 Abstract It is widely accepted that mitochondrial DNA (mtDNA) control region evolves faster than protein encoding genes with few excep- tions. In the present study, we sequenced the mitochondrial cytochrome b gene (cyt b) and control region (CR) and compared their rates in 93 specimens representing 67 species of loaches and some related taxa in the Cobitoidea (Order Cypriniformes). The results showed that sequence divergences of the CR were broadly higher than those of the cyt b (about 1.83 times). However, in considering only closely related species, CR sequence evolution was slower than that of cyt b gene (ratio of CR/cyt b is 0.78), a pattern that is found to be very common in Cypriniformes. Combined data of the cyt b and CR were used to estimate the phylogenetic relationship of the Cobitoidea by maximum parsimony, neighbor-joining, and Bayesian methods. -

A Functional-Morphological Study on the Attachment, Respiration and Feeding Mechanisms in Balitorinae (Balitoridae, Teleostei)
Faculty of Sciences Department of Biology Research group: Evolutionary Morphology of Vertebrates Academic year 2012-2013 A functional-morphological study on the attachment, respiration and feeding mechanisms in Balitorinae (Balitoridae, Teleostei) De Meyer Jens Supervisor: Dr. Tom Geerinckx Thesis submitted to obtain the degree of Tutor: Dr. Tom Geerinckx Master in Biology II © Faculty of Sciences – Evolutionary Morphology of Vertebrates Deze masterproef bevat vertrouwelijk informatie en vertrouwelijke onderzoeksresultaten die toebehoren aan de UGent. De inhoud van de masterproef mag onder geen enkele manier publiek gemaakt worden, noch geheel noch gedeeltelijk zonder de uitdrukkelijke schriftelijke voorafgaandelijke toestemming van de UGent vertegenwoordiger, in casu de promotor. Zo is het nemen van kopieën of het op eender welke wijze dupliceren van het eindwerk verboden, tenzij met schriftelijke toestemming. Het niet respecteren van de confidentiële aard van het eindwerk veroorzaakt onherstelbare schade aan de UGent. Ingeval een geschil zou ontstaan in het kader van deze verklaring, zijn de rechtbanken van het arrondissement Gent uitsluitend bevoegd daarvan kennis te nemen. All rights reserved. This thesis contains confidential information and confidential research results that are property to the UGent. The contents of this master thesis may under no circumstances be made public, nor complete or partial, without the explicit and preceding permission of the UGent representative, i.e. the supervisor. The thesis may under no circumstances be copied or duplicated in any form, unless permission granted in written form. Any violation of the confidential nature of this thesis may impose irreparable damage to the UGent. In case of a dispute that may arise within the context of this declaration, the Judicial Court of© All rights reserved. -

Carp (No Common Name) (Labeo Longipinnis)
Labeo longipinnis (a carp, no common name) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, April 2012 Revised, April 2018 Web Version, 5/16/2018 Photo: J. P. Sullivan. Licensed under CC BY-NC-SA 3.0. Available: http://eol.org/data_objects/17785833. (April 2018). 1 Native Range and Status in the United States Native Range From Moelants (2010): “This species is known from Central Africa Republic to Angola, and Tanzania.” “Central Africa: Labeo longipinnis is known from throughout the Congo River Basin.” “Eastern Africa: One record has been made from Lake Tanganyika (Reid 1985).” “Native: Angola (Angola); Central African Republic; Congo, The Democratic Republic of the; Tanzania, United Republic of” From Froese and Pauly (2018): “Africa: widespread in the Congo River basin, Luapula-Mweru excluded [Tshibwabwa 1997]. Single record from Lake Tanganyika [Reid 1985] unconfirmed [Tshibwabwa 1997] and doubtful.” 1 Status in the United States This species has not been reported as introduced or established in the U.S. There is no indication that this species is in trade in the U.S. Means of Introductions in the United States This species has not been reported as introduced or established in the U.S. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2018): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Actinopterygii Class Teleostei Superorder Ostariophysi Order Cypriniformes Superfamily Cyprinoidea Family Cyprinidae -

Table 7: Species Changing IUCN Red List Status (2018-2019)
IUCN Red List version 2019-3: Table 7 Last Updated: 10 December 2019 Table 7: Species changing IUCN Red List Status (2018-2019) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2018 (IUCN Red List version 2018-2) and 2019 (IUCN Red List version 2019-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2018) List (2019) change version Category -

Hemibarbus Labeo) Ecological Risk Screening Summary
Barbel Steed (Hemibarbus labeo) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, August 2012 Revised, February 2017 Web Version, 1/14/2018 Photo: Chinese Academy of Fishery Sciences. Licensed under CC BY-NC 3.0. Available: http://fishbase.org/photos/PicturesSummary.php?StartRow=0&ID=17301&what=species&TotRe c=9. (February 2017). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2016): “Asia: throughout the Amur basin [Berg 1964]; eastern Asia from the Amur basin to northern Vietnam, Japan and islands of Hainan and Taiwan [Reshetnikov et al. 1997].” Status in the United States This species has not been reported in the United States. 1 Means of Introductions in the United States This species has not been reported in the United States. Remarks From CABI (2017): “Other Scientific Names Acanthogobio oxyrhynchus Nikolskii, 1903 Barbus labeo Pallas, 1776 Barbus schlegelii Günther, 1868 Cyprinus labeo Pallas, 1776 Gobio barbus Temminck & Schlegel, 1846 Gobiobarbus labeo Pallas, 1776 Hemibarbus barbus Temminck & Schlegel, 1846 Hemibarbus longianalis Kimura, 1934 Pseudogobio chaoi Evermann & Shaw, 1927” 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2017): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Osteichthyes Class Actinopterygii Subclass Neopterygii Infraclass Teleostei Superorder Ostariophysi Order Cypriniformes Superfamily Cyprinoidea Family Cyprinidae Genus Hemibarbus Bleeker, 1860 Species Hemibarbus labeo (Pallas, 1776)” “Taxonomic Status: valid” 2 Size, Weight, and Age Range From Froese and Pauly (2016): “Max length : 62.0 cm TL male/unsexed; [Novikov et al. 2002]; common length : 33.0 cm TL male/unsexed; [Berg 1964]; common length :40.6 cm TL (female); max. -

Four New Records of Fish Species (Cypriniformes: Nemacheilidae
Zoological Research 35 (1): 51−58 DOI:10.11813/j.issn.0254-5853.2014.1.051 Four new records of fish species (Cypriniformes: Nemacheilidae, Balitoridae; Characiformes: Prochilodontidae) and corrections of two misidentified fish species (Tetraodontiformes: Tetraodontidae; Beloniformes: Belonidae) in Yunnan, China Marco Endruweit* Qingshan Road 601, Qingdao, China Abstract: In this study, six fish species of five families are reported for the first time from Yunnan Province, China. The nemacheilid Schistura amplizona Kottelat, 2000 is reported from the Luosuojiang River and Nanlahe River subbasins, Mekong basin; the prochilodontid Prochilodus lineatus (Valenciennes, 1837), the balitorid Vanmanenia serrilineata Kottelat, 2000, and the tetraodontid Monotrete turgidus Kottelat, 2000, from Nanlahe River subbasin, Mekong basin; the balitorid Beaufortia daon (Mai, 1978), and the belonid Xenentodon canciloides (Bleeker, 1854), both, from Black River subbasin, Red River basin. The freshwater puffer M. turgidus and the needlefish X. canciloides have been previously misidentified as Tetraodon leiurus (Bleeker, 1950) and Tylosurus strongylurus (van Hasselt, 1823), respectively. Keywords: New record; Misidentification; Mekong basin; Red River; Yunnan Yunnan Province is located in the Southwest within Chen et al in 1989, respectively 1990 for the second the People’s Republic of China. Its name refers to its volume, giving 226 species and subspecies accounts in location south of the Yunling Mountain range. It shares the first volume plus an additional 173 in the second. international border with Myanmar in the West and Through extensive fieldwork and re-evaluation of Southwest, with Laos and Vietnam in the South; national institutionally stored lots the number of Yunnanese fish borders with Xizang Autonomous Region to the species is growing (for e.g. -
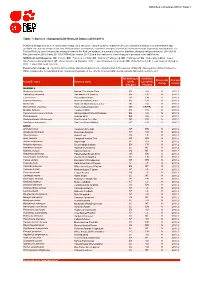
Table 7: Species Changing IUCN Red List Status (2010-2011)
IUCN Red List version 2011.2: Table 7 Table 7: Species changing IUCN Red List Status (2010-2011) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2010 (IUCN Red List version 2010.4) and 2011 (IUCN Red List version 2011.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2010) List (2011) change version Category Category MAMMALS Bradypus torquatus Maned Three-toed Sloth EN VU N 2011.1 Callicebus oenanthe San Martin Titi Monkey EN CR N 2011.1 Equus ferus Przewalski's Horse CR EN G 2011.2 -

Evolution and Phylogenetic Application of the MC1R Gene in the Cobitoidea (Teleostei: Cypriniformes)
ZOOLOGICAL RESEARCH Evolution and phylogenetic application of the MC1R gene in the Cobitoidea (Teleostei: Cypriniformes) Qiong-Ying TANG1,*, Li-Xia SHI1,2, Fei LIU1, Dan YU1, Huan-Zhang LIU1,* 1 The Key Laboratory of Aquatic Biodiversity and Conservation of Chinese Academy of Sciences, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China 2 University of Chinese Academy of Sciences, Beijing 100049, China ABSTRACT INTRODUCTION Fish of the superfamily Cobitoidea sensu stricto (namely loaches) exhibit extremely high diversity of The superfamily Cobitoidea is a group of small- to medium- color patterns, but so far little is known about their sized benthic fish, composed of approximately 28% of species evolutionary mechanism. Melanocortin 1 receptor of the order Cypriniformes, which is the largest group of gene (MC1R) plays an important role during the freshwater fish in the world (Nelson et al., 2016). Depending on synthesis of melanin and formation of animal body different authors, Cobitoidea includes variable families. Bohlen color patterns. In this study, we amplified and sequenced the partial MC1R gene for 44 loach & Šlechtová (2009) and Chen et al. (2009) congruently individuals representing 31 species of four families. recognized the genus Ellopostoma as a distinct new family Phylogenetic analyses yielded a topology congruent Ellopostomatidae, and proposed that Cobitoidea is composed with previous studies using multiple nuclear loci, of eight families (Catostomidae, Gyrinocheilidae, Botiidae, showing that each of the four families was Vaillantellidae, Cobitidae, Ellopostomatidae, Nemacheilidae and monophyletic with sister relationships of Botiidae+ Balitoridae). Kottelat (2012) raised genera Serpenticobitis and (Cobitidae+(Balitoridae+Nemacheilidae)). Gene Barbucca to family rank, and established Serpenticobitidae and evolutionary analyses indicated that MC1R in Barbuccidae.