Rainbow Sharkminnow (Epalzeorhynchos Frenatum) ERSS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Aliens; a Catastrophe for Native Fresh Water Fish Diversity in Pakistan
The Journal of Animal and Plant Sciences, 21(2 Suppl.): 2011, Page: 435-440 ISSN: 1018-7081 ALIENS; A CATASTROPHE FOR NATIVE FRESH WATER FISH DIVERSITY IN PAKISTAN A. M. Khan, Z. Ali, S. Y. Shelly* Z. Ahmad** and M. R. Mirza** Department of Zoology, University of the Punjab, Lahore *Department of Fisheries, Government of Punjab, Munawan, Lahore. Department of Zoology, Government College University, Lahore Corresponding author e-mail: [email protected] ABSTRACT Pakistan has introduced several alien exotic fish species e.g. grass carp (Ctenopharyngodon idella), bighead carp, (Hypophthalmichthys nobilis), silver carp, (Hypophthalmichthys molitrix), common carp (Cyprinus carpio), gold fish (Carassius auratus), and three species of tilapia (Oreochromis aureus, Oreochromis mossambicus, Oreochromis niloticus) in warm waters along with two trout species: the rainbow trout (Onchorynchus mykiss) and the brown trout (Salmo trutta fario) in colder regions for specific purposes like sport fishing, yield enhancement and biological control of aquatic weeds and mosquitoes. The exotic species are becoming invasive in the freshwater biomes of the Punjab and other provinces of Pakistan by reason of their potent reproductive potential and feeding competitions with the native freshwater fish fauna. Resultantly the native fish species viz; Channa marulius, Wallago attu, Rita rita, Sperata sarwari, Gibelion catla, Cirrhinus mrigala and Labeo rohita, which are of economic value are under threat. Key words: Exotic, invasions, freshwater, fish fauna, Pakistan. wild, 421 (35 %) are reported as not established and 177 INTRODUCTION (15 %) with unknown establishment (Fish base, 2003). In Asia, there have been 406 introduction There are more than 186 freshwater fish species records, 176 (43.3 %) are reported as having been described from freshwater bodies of Pakistan. -

Epalzeorhynchos Munense ERSS
Epalzeorhynchos munense (a fish, no common name) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, June 2012 Revised, November 2018 Web Version, 2/1/2019 Photo: W. J. Rainboth/FAO. Licensed under Creative Commons BY-NC 3.0 Unported. Available: http://www.fishbase.org/photos/PicturesSummary.php?StartRow=0&ID=27163&what=species& TotRec=2. (November 9, 2018). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2018a): “Asia: Mekong [Cambodia, Thailand, Laos] and Chao Phraya [Thailand] basins [Kottelat 2001] and Maeklong basin [Thailand] [Vidthayanon et al. 1997].” “[In Laos:] Known from the middle Xe Bangfai, a tributary of the Mekong basin [Kottelat 1998].” 1 Status in the United States No records of Epalzeorhynchos munense in the wild or in trade in the United States were found. Means of Introductions in the United States No records of Epalzeorhynchos munense in the wild in the United States were found. Remarks Information searches were conducted using the valid name Epalzeorhynchos munense and the synonym Labeo munensis. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing According to Fricke et al. (2018), Epalzeorhynchos munense is the valid name for this species. It was originally described as Labeo munensis. From Froese and Pauly (2018b): “Animalia (Kingdom) > Chordata (Phylum) > Vertebrata (Subphylum) > Gnathostomata (Superclass) > […] Actinopterygii (Class) > Cypriniformes (Order) > Cyprinidae (Family) > Epalzeorhynchos munense (Species)” Size, Weight, and Age Range From Froese and Pauly (2018a): “Max length : 9.3 cm SL male/unsexed; [Kottelat 1998]” Environment From Froese and Pauly (2018a): “Freshwater; benthopelagic.” Climate/Range From Froese and Pauly (2018a): “Tropical” 2 Distribution Outside the United States Native From Froese and Pauly (2018a): “Asia: Mekong [Cambodia, Thailand, Laos] and Chao Phraya [Thailand] basins [Kottelat 2001] and Maeklong basin [Thailand] [Vidthayanon et al. -

Induction of Maturation and Ovulation of Red Fin Shark Fish
International Journal of Fisheries and Aquatic Studies 2017; 5(4): 418-424 E-ISSN: 2347-5129 P-ISSN: 2394-0506 (ICV-Poland) Impact Value: 5.62 Induction of maturation and ovulation of Red Fin (GIF) Impact Factor: 0.549 IJFAS 2017; 5(4): 418-424 Shark fish Epalzeorhynchos frenatus in non-spawning © 2017 IJFAS www.fisheriesjournal.com season Received: 03-05-2017 Accepted: 04-06-2017 Muhammad Faiz Islami, Agus Oman Sudrajat and Odang Carman Muhammad Faiz Islami Department of Aquaculture, Bogor Agricultural University, Abstract Bogor, Indonesia, Jl. Agatis There are constraints on the production of red fin shark Epalzeorhynchos frenatus due to the lack of Kampus Dramaga, Bogor, matured broodstocks in Non-spawning season and has a dependence on hormone induction during Indonesia spawning process, because it can’t spawn naturally in cultivation environments. Gonad maturation can be stimulated by the addition of hormones and potential plant-based materials to feed. Thus, the present Agus Oman Sudrajat study attempted alternative hormonal combination to induce both ovulation and spawning in order to Department of Aquaculture, provide farmers with alternative choices for reproduction. This research was divided into two sub- Bogor Agricultural University, research (maturation and ovulation) in one main title. The first section of the study dealt with Bogor, Indonesia, Jl. Agatis maturational induction and it used Completely Randomized Design with 5 treatments. Ten broodstocks Kampus Dramaga, Bogor, were used in each treatment which was as follows: Oodev (0.25 and 0.5 mL. kg-1 fish) addition to feed, Indonesia turmeric powder (250 and 500 mg 100 g-1 feed), and commercial feed (at least 30 % protein) as the control. -

Carp (No Common Name) (Labeo Longipinnis)
Labeo longipinnis (a carp, no common name) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, April 2012 Revised, April 2018 Web Version, 5/16/2018 Photo: J. P. Sullivan. Licensed under CC BY-NC-SA 3.0. Available: http://eol.org/data_objects/17785833. (April 2018). 1 Native Range and Status in the United States Native Range From Moelants (2010): “This species is known from Central Africa Republic to Angola, and Tanzania.” “Central Africa: Labeo longipinnis is known from throughout the Congo River Basin.” “Eastern Africa: One record has been made from Lake Tanganyika (Reid 1985).” “Native: Angola (Angola); Central African Republic; Congo, The Democratic Republic of the; Tanzania, United Republic of” From Froese and Pauly (2018): “Africa: widespread in the Congo River basin, Luapula-Mweru excluded [Tshibwabwa 1997]. Single record from Lake Tanganyika [Reid 1985] unconfirmed [Tshibwabwa 1997] and doubtful.” 1 Status in the United States This species has not been reported as introduced or established in the U.S. There is no indication that this species is in trade in the U.S. Means of Introductions in the United States This species has not been reported as introduced or established in the U.S. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2018): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Actinopterygii Class Teleostei Superorder Ostariophysi Order Cypriniformes Superfamily Cyprinoidea Family Cyprinidae -

Table 7: Species Changing IUCN Red List Status (2018-2019)
IUCN Red List version 2019-3: Table 7 Last Updated: 10 December 2019 Table 7: Species changing IUCN Red List Status (2018-2019) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2018 (IUCN Red List version 2018-2) and 2019 (IUCN Red List version 2019-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2018) List (2019) change version Category -

Hemibarbus Labeo) Ecological Risk Screening Summary
Barbel Steed (Hemibarbus labeo) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, August 2012 Revised, February 2017 Web Version, 1/14/2018 Photo: Chinese Academy of Fishery Sciences. Licensed under CC BY-NC 3.0. Available: http://fishbase.org/photos/PicturesSummary.php?StartRow=0&ID=17301&what=species&TotRe c=9. (February 2017). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2016): “Asia: throughout the Amur basin [Berg 1964]; eastern Asia from the Amur basin to northern Vietnam, Japan and islands of Hainan and Taiwan [Reshetnikov et al. 1997].” Status in the United States This species has not been reported in the United States. 1 Means of Introductions in the United States This species has not been reported in the United States. Remarks From CABI (2017): “Other Scientific Names Acanthogobio oxyrhynchus Nikolskii, 1903 Barbus labeo Pallas, 1776 Barbus schlegelii Günther, 1868 Cyprinus labeo Pallas, 1776 Gobio barbus Temminck & Schlegel, 1846 Gobiobarbus labeo Pallas, 1776 Hemibarbus barbus Temminck & Schlegel, 1846 Hemibarbus longianalis Kimura, 1934 Pseudogobio chaoi Evermann & Shaw, 1927” 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2017): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Osteichthyes Class Actinopterygii Subclass Neopterygii Infraclass Teleostei Superorder Ostariophysi Order Cypriniformes Superfamily Cyprinoidea Family Cyprinidae Genus Hemibarbus Bleeker, 1860 Species Hemibarbus labeo (Pallas, 1776)” “Taxonomic Status: valid” 2 Size, Weight, and Age Range From Froese and Pauly (2016): “Max length : 62.0 cm TL male/unsexed; [Novikov et al. 2002]; common length : 33.0 cm TL male/unsexed; [Berg 1964]; common length :40.6 cm TL (female); max. -

Meristic and Morphometric Characteristics of Crossocheilus Diplochilus (Heckel, 1838) from the Poonch Valley of Jammu and Kashmir, India
World Journal of Zoology 9 (3): 184-189, 2014 ISSN 1817-3098 © IDOSI Publications, 2014 DOI: 10.5829/idosi.wjz.2014.9.3.8570 Meristic and Morphometric Characteristics of Crossocheilus diplochilus (Heckel, 1838) from the Poonch Valley of Jammu and Kashmir, India 1Neeraj Kumar Sharma, 22Javaid Iqbal Mir, Nitya Nand Pandey, 2Mohd. Shahbaz Akhtar, 11Amir Bashir and Ravindra Singh 1Department of Zoology, Tehri Campus, Hemwati Nandan Bahuguna Garhwal University, Tehri Garhwal-249199, India 2Directorate of Coldwater Fisheries Research, Anusandhan Bhawan, Bhimtal 263136, India Abstract: The present study aims to describe the meristic and morphometric characteristics of Crossocheilus diplochilus from a tributary of Indus River basin, India. Altogether 41 specimens ranging from 10.0 - 17.0 cm total length (TL) and 12.16 - 41.22 g body weight (BW) were used for the study of the morphometric and meristic characteristics using different local fishing gears. The morphometric characteristics on the head express greater variation in head height (SD=7.46) than those from the body in pre-anal fin length (SD=4.14). The highly correlated body parameter in relation to total length was standard length (r=0.996) and distance from anal fin to caudal fin base was found least correlated (r=0.804) and strong correlations were observed between head length and pre-orbital length (r=0.931) and least correlation between head length and head height (r=0.829). Even though the values of correlation coefficient (r) vary between 0.804 (distance from anal fin to caudal fin base) and 0.996 (standard length), they are all strongly significant (P<0.001). -

Biodiversity Status.Qxp
163 BIODIVERSITY STATUS OF FISHES INHABITING RIVERS OF KERALA (S. INDIA) WITH SPECIAL REFERENCE TO ENDEMISM, THREATS AND CONSERVATION MEASURES Kurup B.M. Radhakrishnan K.V. Manojkumar T.G. School of Industrial Fisheries, Cochin University of Science & Technology, Cochin 682 016, India E-mail: [email protected] ABSTRACT The identification of 175 freshwater fish- es from 41 west flowing and 3 east flowing river systems of Kerala were confirmed. These can be grouped under 106 ornamental and 67 food fish- es. The biodiversity status of these fishes was assessed according to IUCN criteria. The results showed that populations of the majority of fish species showed drastic reduction over the past five decades. Thirty-three fish species were found to be endemic to the rivers of Kerala. The distributions of the species were found to vary within and between the river systems and some of the species exhibited a high degree of habitat specificity. The diversity and abundance of the species generally showed an inverse relationship with altitude. The serious threats faced by the freshwater fishes of Kerala are mostly in the form of human interventions and habitat alter- ations and conservation plans for the protection and preservation of the unique and rare fish bio- diversity of Kerala are also highlighted. 164 Biodiversity status of fishes inhabiting rivers of Kerala (S.India) INTRODUCTION river. Habitat diversity was given foremost importance during selection of locations within the river system. Kerala is a land of rivers which harbour a rich The sites for habitat inventory were selected based on and diversified fish fauna characterized by many rare channel pattern, channel confinement, gradient and and endemic fish species. -

Ichthyofaunal Diversity and Conservation Status in Rivers of Khyber Pakhtunkhwa, Pakistan
Proceedings of the International Academy of Ecology and Environmental Sciences, 2020, 10(4): 131-143 Article Ichthyofaunal diversity and conservation status in rivers of Khyber Pakhtunkhwa, Pakistan Mukhtiar Ahmad1, Abbas Hussain Shah2, Zahid Maqbool1, Awais Khalid3, Khalid Rasheed Khan2, 2 Muhammad Farooq 1Department of Zoology, Govt. Post Graduate College, Mansehra, Pakistan 2Department of Botany, Govt. Post Graduate College, Mansehra, Pakistan 3Department of Zoology, Govt. Degree College, Oghi, Pakistan E-mail: [email protected] Received 12 August 2020; Accepted 20 September 2020; Published 1 December 2020 Abstract Ichthyofaunal composition is the most important and essential biotic component of an aquatic ecosystem. There is worldwide distribution of fresh water fishes. Pakistan is blessed with a diversity of fishes owing to streams, rivers, dams and ocean. In freshwater bodies of the country about 193 fish species were recorded. There are about 30 species of fish which are commercially exploited for good source of proteins and vitamins. The fish marketing has great socio economic value in the country. Unfortunately, fish fauna is declining at alarming rate due to water pollution, over fishing, pesticide use and other anthropogenic activities. Therefore, about 20 percent of fish population is threatened as endangered or extinct. All Mashers are ‘endangered’, notably Tor putitora, which is also included in the Red List Category of International Union for Conservation of Nature (IUCN) as Endangered. Mashers (Tor species) are distributed in Southeast Asian and Himalayan regions including trans-Himalayan countries like Pakistan and India. The heavy flood of July, 2010 resulted in the minimizing of Tor putitora species Khyber Pakhtunkhwa and the fish is now found extinct from river Swat. -
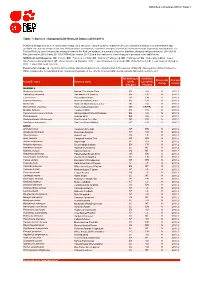
Table 7: Species Changing IUCN Red List Status (2010-2011)
IUCN Red List version 2011.2: Table 7 Table 7: Species changing IUCN Red List Status (2010-2011) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2010 (IUCN Red List version 2010.4) and 2011 (IUCN Red List version 2011.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2010) List (2011) change version Category Category MAMMALS Bradypus torquatus Maned Three-toed Sloth EN VU N 2011.1 Callicebus oenanthe San Martin Titi Monkey EN CR N 2011.1 Equus ferus Przewalski's Horse CR EN G 2011.2 -

Labeo Capensis (Orange River Mudfish) Ecological Risk Screening Summary
Orange River Mudfish (Labeo capensis) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, 2014 Revised, May and July 2019 Web Version, 9/19/2019 Image: G. A. Boulenger. Public domain. Available: https://archive.org/stream/catalogueoffres01brit/catalogueoffres01brit. (July 2019). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2019): “Africa: within the drainage basin of the Orange-Vaal River system [located in Lesotho, Namibia, and South Africa] to which it is possibly restricted. Hitherto thought to occur in the Limpopo system and in southern Cape watersheds [South Africa] which records may be erroneous.” From Barkhuizen et al. (2017): “Native: Lesotho; Namibia; South Africa (Eastern Cape Province - Introduced, Free State, Gauteng, Mpumalanga, Northern Cape Province, North-West Province)” 1 Status in the United States This species has not been reported as introduced or established in the United States. There is no indication that this species is in trade in the United States. Means of Introductions in the United States This species has not been reported as introduced or established in the United States. Remarks A previous version of this ERSS was published in 2014. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2019): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Actinopterygii Class Teleostei Superorder Ostariophysi Order Cypriniformes Superfamily Cyprinoidea Family Cyprinidae Genus Labeo Species Labeo capensis (Smith, 1841)” From Fricke et al. (2019): “Current status: Valid as Labeo capensis (Smith 1841). Cyprinidae: Labeoninae.” Size, Weight, and Age Range From Froese and Pauly (2019): “Max length : 50.0 cm FL male/unsexed; [de Moor and Bruton 1988]; common length : 45.0 cm FL male/unsexed; [Lévêque and Daget 1984]; max.