Lack of Parthenogenesis by Amblyomma Cajennense (Acari
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TICKS in RELATION to HUMAN DISEASES CAUSED by <I
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Navy Research U.S. Department of Defense 1967 TICKS IN RELATION TO HUMAN DISEASES CAUSED BY RICKETTSIA SPECIES Harry Hoogstraal Follow this and additional works at: https://digitalcommons.unl.edu/usnavyresearch This Article is brought to you for free and open access by the U.S. Department of Defense at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in U.S. Navy Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. TICKS IN RELATION TO HUMAN DISEASES CAUSED BY RICKETTSIA SPECIES1,2 By HARRY HOOGSTRAAL Department oj Medical Zoology, United States Naval Medical Research Unit Number Three, Cairo, Egypt, U.A.R. Rickettsiae (185) are obligate intracellular parasites that multiply by binary fission in the cells of both vertebrate and invertebrate hosts. They are pleomorphic coccobacillary bodies with complex cell walls containing muramic acid, and internal structures composed of ribonucleic and deoxyri bonucleic acids. Rickettsiae show independent metabolic activity with amino acids and intermediate carbohydrates as substrates, and are very susceptible to tetracyclines as well as to other antibiotics. They may be considered as fastidious bacteria whose major unique character is their obligate intracellu lar life, although there is at least one exception to this. In appearance, they range from coccoid forms 0.3 J.I. in diameter to long chains of bacillary forms. They are thus intermediate in size between most bacteria and filterable viruses, and form the family Rickettsiaceae Pinkerton. They stain poorly by Gram's method but well by the procedures of Macchiavello, Gimenez, and Giemsa. -

Ehrlichiosis and Anaplasmosis Are Tick-Borne Diseases Caused by Obligate Anaplasmosis: Intracellular Bacteria in the Genera Ehrlichia and Anaplasma
Ehrlichiosis and Importance Ehrlichiosis and anaplasmosis are tick-borne diseases caused by obligate Anaplasmosis: intracellular bacteria in the genera Ehrlichia and Anaplasma. These organisms are widespread in nature; the reservoir hosts include numerous wild animals, as well as Zoonotic Species some domesticated species. For many years, Ehrlichia and Anaplasma species have been known to cause illness in pets and livestock. The consequences of exposure vary Canine Monocytic Ehrlichiosis, from asymptomatic infections to severe, potentially fatal illness. Some organisms Canine Hemorrhagic Fever, have also been recognized as human pathogens since the 1980s and 1990s. Tropical Canine Pancytopenia, Etiology Tracker Dog Disease, Ehrlichiosis and anaplasmosis are caused by members of the genera Ehrlichia Canine Tick Typhus, and Anaplasma, respectively. Both genera contain small, pleomorphic, Gram negative, Nairobi Bleeding Disorder, obligate intracellular organisms, and belong to the family Anaplasmataceae, order Canine Granulocytic Ehrlichiosis, Rickettsiales. They are classified as α-proteobacteria. A number of Ehrlichia and Canine Granulocytic Anaplasmosis, Anaplasma species affect animals. A limited number of these organisms have also Equine Granulocytic Ehrlichiosis, been identified in people. Equine Granulocytic Anaplasmosis, Recent changes in taxonomy can make the nomenclature of the Anaplasmataceae Tick-borne Fever, and their diseases somewhat confusing. At one time, ehrlichiosis was a group of Pasture Fever, diseases caused by organisms that mostly replicated in membrane-bound cytoplasmic Human Monocytic Ehrlichiosis, vacuoles of leukocytes, and belonged to the genus Ehrlichia, tribe Ehrlichieae and Human Granulocytic Anaplasmosis, family Rickettsiaceae. The names of the diseases were often based on the host Human Granulocytic Ehrlichiosis, species, together with type of leukocyte most often infected. -

An Insight Into the Ecobiology, Vector Significance and Control of Hyalomma Ticks (Acari: Ixodidae): a Review
Accepted Manuscript Title: AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW Authors: M.S. Sajid, A. Kausar, A. Iqbal, H. Abbas, Z. Iqbal, M.K. Jones PII: S0001-706X(18)30862-3 DOI: https://doi.org/10.1016/j.actatropica.2018.08.016 Reference: ACTROP 4752 To appear in: Acta Tropica Received date: 6-7-2018 Revised date: 10-8-2018 Accepted date: 12-8-2018 Please cite this article as: Sajid MS, Kausar A, Iqbal A, Abbas H, Iqbal Z, Jones MK, AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW, Acta Tropica (2018), https://doi.org/10.1016/j.actatropica.2018.08.016 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW M. S. SAJID 1 2 *, A. KAUSAR 3, A. IQBAL 4, H. ABBAS 5, Z. IQBAL 1, M. K. JONES 6 1. Department of Parasitology, Faculty of Veterinary Science, University of Agriculture, Faisalabad-38040, Pakistan. 2. One Health Laboratory, Center for Advanced Studies in Agriculture and Food Security (CAS-AFS) University of Agriculture, Faisalabad-38040, Pakistan. -

Arthropod Parasites in Domestic Animals
ARTHROPOD PARASITES IN DOMESTIC ANIMALS Abbreviations KINGDOM PHYLUM CLASS ORDER CODE Metazoa Arthropoda Insecta Siphonaptera INS:Sip Mallophaga INS:Mal Anoplura INS:Ano Diptera INS:Dip Arachnida Ixodida ARA:Ixo Mesostigmata ARA:Mes Prostigmata ARA:Pro Astigmata ARA:Ast Crustacea Pentastomata CRU:Pen References Ashford, R.W. & Crewe, W. 2003. The parasites of Homo sapiens: an annotated checklist of the protozoa, helminths and arthropods for which we are home. Taylor & Francis. Taylor, M.A., Coop, R.L. & Wall, R.L. 2007. Veterinary Parasitology. 3rd edition, Blackwell Pub. HOST-PARASITE CHECKLIST Class: MAMMALIA [mammals] Subclass: EUTHERIA [placental mammals] Order: PRIMATES [prosimians and simians] Suborder: SIMIAE [monkeys, apes, man] Family: HOMINIDAE [man] Homo sapiens Linnaeus, 1758 [man] ARA:Ast Sarcoptes bovis, ectoparasite (‘milker’s itch’)(mange mite) ARA:Ast Sarcoptes equi, ectoparasite (‘cavalryman’s itch’)(mange mite) ARA:Ast Sarcoptes scabiei, skin (mange mite) ARA:Ixo Ixodes cornuatus, ectoparasite (scrub tick) ARA:Ixo Ixodes holocyclus, ectoparasite (scrub tick, paralysis tick) ARA:Ixo Ornithodoros gurneyi, ectoparasite (kangaroo tick) ARA:Pro Cheyletiella blakei, ectoparasite (mite) ARA:Pro Cheyletiella parasitivorax, ectoparasite (rabbit fur mite) ARA:Pro Demodex brevis, sebacceous glands (mange mite) ARA:Pro Demodex folliculorum, hair follicles (mange mite) ARA:Pro Trombicula sarcina, ectoparasite (black soil itch mite) INS:Ano Pediculus capitis, ectoparasite (head louse) INS:Ano Pediculus humanus, ectoparasite (body -

Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences
Georgia Southern University Digital Commons@Georgia Southern Electronic Theses and Dissertations Graduate Studies, Jack N. Averitt College of Spring 2009 Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences Erica Janelle Burkman Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/etd Recommended Citation Burkman, Erica Janelle, "Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences" (2009). Electronic Theses and Dissertations. 704. https://digitalcommons.georgiasouthern.edu/etd/704 This thesis (open access) is brought to you for free and open access by the Graduate Studies, Jack N. Averitt College of at Digital Commons@Georgia Southern. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. GENETIC STRUCTURE OF AMBLYOMMA CAJENNENSE (ACARI: IXODIDAE) POPULATIONS BASED ON MITOCHONDRIAL GENE SEQUENCES by ERICA JANELLE BURKMAN (Under the Direction of Dr. Lorenza Beati) ABSTRACT Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) is a common tick species that has a large geographic distribution from the southern regions of the United States (Texas), to the Caribbean Islands, Central, and South America. This tick is a vector of the agent of Brazilian spotted fever, an often fatal disease in South America. Throughout its geographic range, populations of A. cajennense have shown differences in ecological adaptation while feeding on a variety of hosts ranging from livestock, birds, and humans. In order to examine the taxonomic status and phylogeographic evolution of this species, we analyzed mitochondrial 12S rDNA, control region (d-loop), and cytochrome oxidase II gene sequences of A. -

Diapause and Quiescence As Two Main Kinds of Dormancy and Their Significance in Life Cycles of Mites and Ticks (Chelicerata: Arachnida: Acari)
Acarina 17 (1): 3–32 © Acarina 2009 DIAPAUSE AND QUIESCENCE AS TWO MAIN KINDS OF DORMANCY AND THEIR SIGNIFICANCE IN LIFE CYCLES OF MITES AND TICKS (CHELICERATA: ARACHNIDA: ACARI). PART 2. PARASITIFORMES V. N. Belozerov Biological Research Institute, St. Petersburg State University, Peterhof 198504, Russia; e-mail: [email protected] ABSTRACT: Concerning the problem of life history and such an important its aspect as seasonality of life cycles and their control enabled by dormant stages, the parasitiform mites reveal the obvious similarity with the acariform mites. This concerns the pres- ence of both main kinds of dormancy (diapause and quiescence). The great importance in the seasonal control of life cycles in some parasitiform mites, like in acariform mites, belongs also for combinations of diapause with non-diapause arrests, particularly with the post-diapause quiescence (PDQ). This type of quiescence evoked after termination of diapause and enabling more accu- rate time-adjustment in recommencement of active development, is characteristic of both lineages of the Parasitiformes — Ixodida and Mesostigmata (particularly Gamasida). The available data show that in ixodid ticks the PDQ may be resulted similarly after developmental and behavioral diapause. Reproductive diapause combined with the PDQ is characteristic of some gamasid mites (particularly the family Phytoseiidae), while most gamasid and uropodid mites with phoretic dispersal reveal the dormant state (apparently of diapause nature) at the deutonymphal stage. The uncertainty between diapause and non-diapause dormancy is retained in some many cases (even in ixodid ticks and phytoseiid mites), and the necessity of further thorough study of different forms of diapause and non-diapause arrests in representatives of the Acari is noted therefore. -

Morphometrics of Amblyomma Mixtum in the State of Veracruz, Mexico
pathogens Article Morphometrics of Amblyomma mixtum in the State of Veracruz, Mexico Mariel Aguilar-Domínguez 1, Dora Romero-Salas 1,* , Sokani Sánchez-Montes 2, Ricardo Serna-Lagunes 3 , Greta Rosas-Saito 4, Anabel Cruz-Romero 1 and Adalberto A. Pérez de León 5,6 1 Laboratorio de Parasitología, rancho “Torreón del Molino”, Facultad de Medicina Veterinaria y Zootecnia, Universidad Veracruzana, Veracruz 91697, Mexico; [email protected] (M.A.-D.); [email protected] (A.C.-R.) 2 Facultad de Ciencias Biológicas y Agropecuarias Región Tuxpan, Universidad Veracruzana, Tuxpam 92870, Mexico; [email protected] 3 Laboratorio de Bioinformática y Bioestadística, Facultad de Ciencias Biológicas y Agropecuarias, Universidad Veracruzana, Córdoba 94945, Mexico; [email protected] 4 Red de Estudios Moleculares Avanzados, Instituto de Ecología, Xalapa 91073, Mexico; [email protected] 5 USDA-ARS Knipling-Bushland U.S. Veterinary Pest Genomics Center and Livestock Insects Research Laboratory, Kerrville, TX 78028, USA; [email protected] 6 USDA-ARS San Joaquin Valley Agricultural Sciences Center, Parlier, CA 93648, USA * Correspondence: [email protected]; Tel.: +52-(229)-9342075 Abstract: The tick Amblyomma mixtum is an ectoparasite of veterinary and public health importance because of its role as a vector of zoonotic pathogens. However, little is known about A. mixtum intraspecific variability and if morphological differentiation exists between populations across its geographic range. This study aimed to determine by electron microscopy the morphological vari- ability of A. mixtum populations in the state of Veracruz, which has a large livestock population Citation: Aguilar-Domínguez, M.; among states in Mexico. Forty male and 40 female A. mixtum collected from the 10 natural regions Romero-Salas, D.; Sánchez-Montes, S.; of Veracruz state were analyzed microscopically to accomplish main component analysis for each Serna-Lagunes, R.; Rosas-Saito, G.; sex. -

Annotated Bibliography for Barrow Island Terrestrial Invertebrates
RECORDS OF THE WESTERN AUSTRALIAN MUSEUM 83 135–144 (2013) DOI: 10.18195/issn.0313-122x.83.2013.135-144 SUPPLEMENT Annotated bibliography for Barrow Island terrestrial invertebrates Christopher K. Taylor Department of Environment and Agriculture, Curtin University, GPO Box U1987, Perth, Western Australia 6845, Australia. Email: [email protected] ABSTRACT – A bibliography is provided of publications treating terrestrial invertebrates on Barrow Island. A brief overview is also given of natural history and invertebrate collections on Barrow Island. KEYWORDS: Arthropoda, Insecta, Arachnida, Gastropoda, publication history INTRODUCTION During the late 1800s Barrow Island was utilised at various times by pastoralists, guano miners, pearl As part of this special issue on the terrestrial and turtle fishers, and slavers (Hook et al. 2004; invertebrate fauna of Barrow Island in Western ‘Supreme Court—Civil Side’, West Australian, 26 Australia, we take the opportunity to present May 1887; ‘The native question’, Daily News [Perth], a bibliography of previous publications on the 16 February 1905). If any of these individuals subject. A more general bibliography of Barrow were interested in collecting invertebrates, their Island’s natural history was previously collated by endeavours in that field have not been recorded for Smith et al. (2006). The current bibliography differs posterity. from that in gathering not only publications for which Barrow Island was the primary focus, but J.T. Tunney of the Western Australian Museum also those in which Barrow Island specimens were spent six weeks on Barrow Island in 1901 (‘News considered as part of a broader study. and notes’, West Australian, 22 March 1901). -

Ehrlichia, and Anaplasma Species in Australian Human-Biting Ticks
RESEARCH ARTICLE Bacterial Profiling Reveals Novel “Ca. Neoehrlichia”, Ehrlichia, and Anaplasma Species in Australian Human-Biting Ticks Alexander W. Gofton1*, Stephen Doggett2, Andrew Ratchford3, Charlotte L. Oskam1, Andrea Paparini1, Una Ryan1, Peter Irwin1* 1 Vector and Water-borne Pathogen Research Group, School of Veterinary and Life Sciences, Murdoch University, Perth, Western Australia, Australia, 2 Department of Medical Entomology, Pathology West and Institute for Clinical Pathology and Medical Research, Westmead Hospital, Westmead, New South Wales, Australia, 3 Emergency Department, Mona Vale Hospital, New South Wales, Australia * [email protected] (AWG); [email protected] (PI) Abstract OPEN ACCESS In Australia, a conclusive aetiology of Lyme disease-like illness in human patients remains Citation: Gofton AW, Doggett S, Ratchford A, Oskam elusive, despite growing numbers of people presenting with symptoms attributed to tick CL, Paparini A, Ryan U, et al. (2015) Bacterial bites. In the present study, we surveyed the microbial communities harboured by human-bit- Profiling Reveals Novel “Ca. Neoehrlichia”, Ehrlichia, ing ticks from across Australia to identify bacteria that may contribute to this syndrome. and Anaplasma Species in Australian Human-Biting Ticks. PLoS ONE 10(12): e0145449. doi:10.1371/ Universal PCR primers were used to amplify the V1-2 hyper-variable region of bacterial journal.pone.0145449 16S rRNA genes in DNA samples from individual Ixodes holocyclus (n = 279), Amblyomma Editor: Bradley S. Schneider, Metabiota, UNITED triguttatum (n = 167), Haemaphysalis bancrofti (n = 7), and H. longicornis (n = 7) ticks. STATES The 16S amplicons were sequenced on the Illumina MiSeq platform and analysed in Received: October 12, 2015 USEARCH, QIIME, and BLAST to assign genus and species-level taxonomies. -

Scoping Review of Distribution Models for Selected Amblyomma Ticks and Rickettsial Group Pathogens
Scoping review of distribution models for selected Amblyomma ticks and rickettsial group pathogens Catherine A. Lippi1,2, Holly D. Gaff3,4, Alexis L. White1,2 and Sadie J. Ryan1,2 1 Department of Geography, University of Florida, Gainesville, FL, USA 2 Emerging Pathogens Institute, University of Florida, Gainesville, FL, USA 3 Department of Biology, Old Dominion University, Norfolk, VA, USA 4 School of Mathematics, Statistics and Computer Science, University of Kwa-Zulu Natal, Durban, South Africa ABSTRACT The rising prevalence of tick-borne diseases in humans in recent decades has called attention to the need for more information on geographic risk for public health planning. Species distribution models (SDMs) are an increasingly utilized method of constructing potential geographic ranges. There are many knowledge gaps in our understanding of risk of exposure to tick-borne pathogens, particularly for those in the rickettsial group. Here, we conducted a systematic scoping review of the SDM literature for rickettsial pathogens and tick vectors in the genus Amblyomma. Of the 174 reviewed articles, only 24 studies used SDMs to estimate the potential extent of vector and/or pathogen ranges. The majority of studies (79%) estimated only tick distributions using vector presence as a proxy for pathogen exposure. Studies were conducted at different scales and across multiple continents. Few studies undertook original data collection, and SDMs were mostly built with presence-only datasets from public database or surveillance sources. The reliance on existing data sources, using ticks as a proxy for disease risk, may simply reflect a lag in new data acquisition and a thorough understanding of the tick-pathogen ecology involved. -
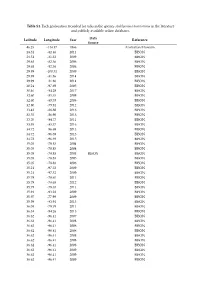
Table S1. Each Geolocation Recorded for Ticks in the Species Amblyomma Americanum in the Literature and Publicly Available Online Databases
Table S1. Each geolocation recorded for ticks in the species Amblyomma americanum in the literature and publicly available online databases. Data Latitude Longitude Year Reference Source 46.25 -114.17 1966 Australian Museum 28.31 -82.46 2011 BISON 28.51 -81.32 2009 BISON 29.68 -82.36 2006 BISON 29.68 -82.36 2006 BISON 29.99 -100.31 2009 BISON 29.99 -81.86 2014 BISON 29.99 -81.86 2014 BISON 30.24 -97.69 2005 BISON 30.46 -84.28 2017 BISON 32.60 -85.35 2008 BISON 32.60 -85.35 2008 BISON 32.80 -79.94 2012 BISON 33.42 -88.88 2016 BISON 33.55 -86.90 2013 BISON 33.70 -84.77 2011 BISON 33.95 -83.37 2016 BISON 34.72 -96.69 2013 BISON 34.72 -96.69 2013 BISON 34.72 -96.69 2015 BISON 35.05 -78.83 2004 BISON 35.05 -78.83 2004 BISON 35.05 -78.83 2004 BISON BISON 35.05 -78.83 2005 BISON 35.05 -78.83 2006 BISON 35.21 -97.32 2009 BISON 35.21 -97.32 2009 BISON 35.79 -78.65 2011 BISON 35.79 -78.65 2012 BISON 35.79 -78.65 2011 BISON 35.91 -93.22 2009 BISON 35.97 -77.99 2009 BISON 35.99 -83.94 2015 BISON 36.08 -79.79 2011 BISON 36.34 -94.26 2015 BISON 36.62 -96.41 2007 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2011 BISON 36.62 -96.41 2012 BISON 36.62 -96.41 2012 BISON 37.36 -77.05 2014 BISON 37.62 -84.87 2016 BISON 37.83 -78.28 2011 BISON 37.97 -85.70 2016 BISON 37.97 -85.70 2016 BISON 38.22 -75.31 2014 BISON 38.43 -88.43 -

The Role of Humans in the Importation of Ticks to New Zealand
THE NEW ZEALAND MEDICAL JOURNAL Journal of the New Zealand Medical Association The role of humans in the importation of ticks to New Zealand: a threat to public health and biosecurity Allen C G Heath, Scott Hardwick Abstract Humans coming into New Zealand occasionally, and unwittingly, bring exotic ticks with them, either attached to their bodies or with luggage. Of the 172 available records for tick interception at New Zealand’s border, half can be attributed to human agency. Here, together with an outline of tick biology and ecology, we present evidence of at least 17 species of ticks being brought in by humans, with Australia, North America and Asia the most frequent countries of origin. Risks posed by some of the nine species of ticks already in New Zealand are briefly examined. Sites of attachment of ticks and associated symptoms where these have been recorded are presented. Diseases transmitted by ticks and most likely to be encountered by travellers are briefly discussed together with the most practical method of tick removal. A plea is made for practitioners to increase their awareness of the risks to New Zealand’s biosecurity and public health posed by ticks and to ensure that as many as possible of these unwelcome ‘souvenirs’ are collected and passed on for identification. The world tick fauna comprises about 900 species of which New Zealand has 11 confirmed. 1 Four of these are endemic (kiwi tick, Ixodes anatis ; tuatara tick, Amblyomma (formerly Aponomma ) sphenodonti and the cormorant tick, I. jacksoni ), as well as a new species of Carios from a native bat, and the others are either exotic (Carios (formerly Ornithodoros ) capensis , Haemaphysalis longicornis, Ixodes amersoni ) or shared with Australia ( Ixodes eudyptidis ), or distributed throughout the sub-Antarctic faunal region ( I.