82179247.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Official Nh Dhhs Health Alert
THIS IS AN OFFICIAL NH DHHS HEALTH ALERT Distributed by the NH Health Alert Network [email protected] May 18, 2018, 1300 EDT (1:00 PM EDT) NH-HAN 20180518 Tickborne Diseases in New Hampshire Key Points and Recommendations: 1. Blacklegged ticks transmit at least five different infections in New Hampshire (NH): Lyme disease, Anaplasma, Babesia, Powassan virus, and Borrelia miyamotoi. 2. NH has one of the highest rates of Lyme disease in the nation, and 50-60% of blacklegged ticks sampled from across NH have been found to be infected with Borrelia burgdorferi, the bacterium that causes Lyme disease. 3. NH has experienced a significant increase in human cases of anaplasmosis, with cases more than doubling from 2016 to 2017. The reason for the increase is unknown at this time. 4. The number of new cases of babesiosis also increased in 2017; because Babesia can be transmitted through blood transfusions in addition to tick bites, providers should ask patients with suspected babesiosis whether they have donated blood or received a blood transfusion. 5. Powassan is a newer tickborne disease which has been identified in three NH residents during past seasons in 2013, 2016 and 2017. While uncommon, Powassan can cause a debilitating neurological illness, so providers should maintain an index of suspicion for patients presenting with an unexplained meningoencephalitis. 6. Borrelia miyamotoi infection usually presents with a nonspecific febrile illness similar to other tickborne diseases like anaplasmosis, and has recently been identified in one NH resident. Tests for Lyme disease do not reliably detect Borrelia miyamotoi, so providers should consider specific testing for Borrelia miyamotoi (see Attachment 1) and other pathogens if testing for Lyme disease is negative but a tickborne disease is still suspected. -

TICKS in RELATION to HUMAN DISEASES CAUSED by <I
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Navy Research U.S. Department of Defense 1967 TICKS IN RELATION TO HUMAN DISEASES CAUSED BY RICKETTSIA SPECIES Harry Hoogstraal Follow this and additional works at: https://digitalcommons.unl.edu/usnavyresearch This Article is brought to you for free and open access by the U.S. Department of Defense at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in U.S. Navy Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. TICKS IN RELATION TO HUMAN DISEASES CAUSED BY RICKETTSIA SPECIES1,2 By HARRY HOOGSTRAAL Department oj Medical Zoology, United States Naval Medical Research Unit Number Three, Cairo, Egypt, U.A.R. Rickettsiae (185) are obligate intracellular parasites that multiply by binary fission in the cells of both vertebrate and invertebrate hosts. They are pleomorphic coccobacillary bodies with complex cell walls containing muramic acid, and internal structures composed of ribonucleic and deoxyri bonucleic acids. Rickettsiae show independent metabolic activity with amino acids and intermediate carbohydrates as substrates, and are very susceptible to tetracyclines as well as to other antibiotics. They may be considered as fastidious bacteria whose major unique character is their obligate intracellu lar life, although there is at least one exception to this. In appearance, they range from coccoid forms 0.3 J.I. in diameter to long chains of bacillary forms. They are thus intermediate in size between most bacteria and filterable viruses, and form the family Rickettsiaceae Pinkerton. They stain poorly by Gram's method but well by the procedures of Macchiavello, Gimenez, and Giemsa. -

Bitten Enhance.Pdf
bitten. Copyright © 2019 by Kris Newby. All rights reserved. Printed in the United States of America. No part of this book may be used or reproduced in any manner whatsoever without written permission except in the case of brief quotations embodied in critical articles and reviews. For information, address HarperCollins Publishers, 195 Broadway, New York, NY 10007. HarperCollins books may be purchased for educational, business, or sales pro- motional use. For information, please email the Special Markets Department at [email protected]. first edition Frontispiece: Tick research at Rocky Mountain Laboratories, in Hamilton, Mon- tana (Courtesy of Gary Hettrick, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]) Maps by Nick Springer, Springer Cartographics Designed by William Ruoto Library of Congress Cataloging- in- Publication Data Names: Newby, Kris, author. Title: Bitten: the secret history of lyme disease and biological weapons / Kris Newby. Description: New York, NY: Harper Wave, [2019] Identifiers: LCCN 2019006357 | ISBN 9780062896278 (hardback) Subjects: LCSH: Lyme disease— History. | Lyme disease— Diagnosis. | Lyme Disease— Treatment. | BISAC: HEALTH & FITNESS / Diseases / Nervous System (incl. Brain). | MEDICAL / Diseases. | MEDICAL / Infectious Diseases. Classification: LCC RC155.5.N49 2019 | DDC 616.9/246—dc23 LC record available at https://lccn.loc.gov/2019006357 19 20 21 22 23 lsc 10 9 8 7 6 5 4 3 2 1 Appendix I: Ticks and Human Disease Agents -

Ehrlichiosis and Anaplasmosis Are Tick-Borne Diseases Caused by Obligate Anaplasmosis: Intracellular Bacteria in the Genera Ehrlichia and Anaplasma
Ehrlichiosis and Importance Ehrlichiosis and anaplasmosis are tick-borne diseases caused by obligate Anaplasmosis: intracellular bacteria in the genera Ehrlichia and Anaplasma. These organisms are widespread in nature; the reservoir hosts include numerous wild animals, as well as Zoonotic Species some domesticated species. For many years, Ehrlichia and Anaplasma species have been known to cause illness in pets and livestock. The consequences of exposure vary Canine Monocytic Ehrlichiosis, from asymptomatic infections to severe, potentially fatal illness. Some organisms Canine Hemorrhagic Fever, have also been recognized as human pathogens since the 1980s and 1990s. Tropical Canine Pancytopenia, Etiology Tracker Dog Disease, Ehrlichiosis and anaplasmosis are caused by members of the genera Ehrlichia Canine Tick Typhus, and Anaplasma, respectively. Both genera contain small, pleomorphic, Gram negative, Nairobi Bleeding Disorder, obligate intracellular organisms, and belong to the family Anaplasmataceae, order Canine Granulocytic Ehrlichiosis, Rickettsiales. They are classified as α-proteobacteria. A number of Ehrlichia and Canine Granulocytic Anaplasmosis, Anaplasma species affect animals. A limited number of these organisms have also Equine Granulocytic Ehrlichiosis, been identified in people. Equine Granulocytic Anaplasmosis, Recent changes in taxonomy can make the nomenclature of the Anaplasmataceae Tick-borne Fever, and their diseases somewhat confusing. At one time, ehrlichiosis was a group of Pasture Fever, diseases caused by organisms that mostly replicated in membrane-bound cytoplasmic Human Monocytic Ehrlichiosis, vacuoles of leukocytes, and belonged to the genus Ehrlichia, tribe Ehrlichieae and Human Granulocytic Anaplasmosis, family Rickettsiaceae. The names of the diseases were often based on the host Human Granulocytic Ehrlichiosis, species, together with type of leukocyte most often infected. -

An Insight Into the Ecobiology, Vector Significance and Control of Hyalomma Ticks (Acari: Ixodidae): a Review
Accepted Manuscript Title: AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW Authors: M.S. Sajid, A. Kausar, A. Iqbal, H. Abbas, Z. Iqbal, M.K. Jones PII: S0001-706X(18)30862-3 DOI: https://doi.org/10.1016/j.actatropica.2018.08.016 Reference: ACTROP 4752 To appear in: Acta Tropica Received date: 6-7-2018 Revised date: 10-8-2018 Accepted date: 12-8-2018 Please cite this article as: Sajid MS, Kausar A, Iqbal A, Abbas H, Iqbal Z, Jones MK, AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW, Acta Tropica (2018), https://doi.org/10.1016/j.actatropica.2018.08.016 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. AN INSIGHT INTO THE ECOBIOLOGY, VECTOR SIGNIFICANCE AND CONTROL OF HYALOMMA TICKS (ACARI: IXODIDAE): A REVIEW M. S. SAJID 1 2 *, A. KAUSAR 3, A. IQBAL 4, H. ABBAS 5, Z. IQBAL 1, M. K. JONES 6 1. Department of Parasitology, Faculty of Veterinary Science, University of Agriculture, Faisalabad-38040, Pakistan. 2. One Health Laboratory, Center for Advanced Studies in Agriculture and Food Security (CAS-AFS) University of Agriculture, Faisalabad-38040, Pakistan. -

Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences
Georgia Southern University Digital Commons@Georgia Southern Electronic Theses and Dissertations Graduate Studies, Jack N. Averitt College of Spring 2009 Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences Erica Janelle Burkman Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/etd Recommended Citation Burkman, Erica Janelle, "Genetic Structure of Amblyomma Cajennense (Acari: Ixodidae) Populations Based on Mitochondrial Gene Sequences" (2009). Electronic Theses and Dissertations. 704. https://digitalcommons.georgiasouthern.edu/etd/704 This thesis (open access) is brought to you for free and open access by the Graduate Studies, Jack N. Averitt College of at Digital Commons@Georgia Southern. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. GENETIC STRUCTURE OF AMBLYOMMA CAJENNENSE (ACARI: IXODIDAE) POPULATIONS BASED ON MITOCHONDRIAL GENE SEQUENCES by ERICA JANELLE BURKMAN (Under the Direction of Dr. Lorenza Beati) ABSTRACT Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae) is a common tick species that has a large geographic distribution from the southern regions of the United States (Texas), to the Caribbean Islands, Central, and South America. This tick is a vector of the agent of Brazilian spotted fever, an often fatal disease in South America. Throughout its geographic range, populations of A. cajennense have shown differences in ecological adaptation while feeding on a variety of hosts ranging from livestock, birds, and humans. In order to examine the taxonomic status and phylogeographic evolution of this species, we analyzed mitochondrial 12S rDNA, control region (d-loop), and cytochrome oxidase II gene sequences of A. -

Tick-Borne Diseases in Maine a Physician’S Reference Manual Deer Tick Dog Tick Lonestar Tick (CDC Photo)
tick-borne diseases in Maine A Physician’s Reference Manual Deer Tick Dog Tick Lonestar Tick (CDC PHOTO) Nymph Nymph Nymph Adult Male Adult Male Adult Male Adult Female Adult Female Adult Female images not to scale know your ticks Ticks are generally found in brushy or wooded areas, near the DEER TICK DOG TICK LONESTAR TICK Ixodes scapularis Dermacentor variabilis Amblyomma americanum ground; they cannot jump or fly. Ticks are attracted to a variety (also called blacklegged tick) (also called wood tick) of host factors including body heat and carbon dioxide. They will Diseases Diseases Diseases transfer to a potential host when one brushes directly against Lyme disease, Rocky Mountain spotted Ehrlichiosis anaplasmosis, babesiosis fever and tularemia them and then seek a site for attachment. What bites What bites What bites Nymph and adult females Nymph and adult females Adult females When When When April through September in Anytime temperatures are April through August New England, year-round in above freezing, greatest Southern U.S. Coloring risk is spring through fall Adult females have a dark Coloring Coloring brown body with whitish Adult females have a brown Adult females have a markings on its hood body with a white spot on reddish-brown tear shaped the hood Size: body with dark brown hood Unfed Adults: Size: Size: Watermelon seed Nymphs: Poppy seed Nymphs: Poppy seed Unfed Adults: Sesame seed Unfed Adults: Sesame seed suMMer fever algorithM ALGORITHM FOR DIFFERENTIATING TICK-BORNE DISEASES IN MAINE Patient resides, works, or recreates in an area likely to have ticks and is exhibiting fever, This algorithm is intended for use as a general guide when pursuing a diagnosis. -

Molecular Evidence of Babesia Infections in Spinose Ear Tick, Otobius Megnini Infesting Stabled Horses in Nuwara Eliya Racecourse: a Case Study
Ceylon Journal of Science 47(4) 2018: 405-409 DOI: http://doi.org/10.4038/cjs.v47i4.7559 SHORT COMMUNICATION Molecular evidence of Babesia infections in Spinose ear tick, Otobius megnini infesting stabled horses in Nuwara Eliya racecourse: A case study G.C.P. Diyes1,2, R.P.V.J. Rajapakse3 and R.S. Rajakaruna1,2,* 1Department of Zoology, Faculty of Science, University of Peradeniya, Peradeniya 20400, Sri Lanka 2The Postgraduate Institute of Science, University of Peradeniya, Peradeniya 20400, Sri Lanka 3Department of Veterinary Pathobiology, Faculty of Veterinary Medicine & Animal Science, University of Peradeniya, Peradeniya 20400, Sri Lanka Received:26/04/2018; Accepted:02/08/2018 Abstract: Spinose ear tick, Otobius megnini (Family Argasidae) Race Club (Joseph, 1982). There is a speculation that O. is a one-host soft tick that parasitizes domesticated animals and megnini was introduced to Sri Lanka from India via horse occasionally humans. It causes otoacariasis or parasitic otitis in trading. The first report of O. megnini in Sri Lanka is in humans and animals and also known to carry infectious agents. 2010 from stable workers and jockeys as an intra-aural Intra aural infestations of O. megnini is a serious health problem infestation (Ariyaratne et al., 2010). In Sri Lanka, O. in the well-groomed race horses in Nuwara Eliya. Otobius megnini appears to have a limited distribution with no megnini collected from the ear canal of stabled horses in Nuwara records of it infesting any other domesticated animals other Eliya racecourse were tested for three possible infections, than horses in the racecourses (Diyes and Rajakaruna, Rickettsia, Theileria and Babesia. -

Morphometrics of Amblyomma Mixtum in the State of Veracruz, Mexico
pathogens Article Morphometrics of Amblyomma mixtum in the State of Veracruz, Mexico Mariel Aguilar-Domínguez 1, Dora Romero-Salas 1,* , Sokani Sánchez-Montes 2, Ricardo Serna-Lagunes 3 , Greta Rosas-Saito 4, Anabel Cruz-Romero 1 and Adalberto A. Pérez de León 5,6 1 Laboratorio de Parasitología, rancho “Torreón del Molino”, Facultad de Medicina Veterinaria y Zootecnia, Universidad Veracruzana, Veracruz 91697, Mexico; [email protected] (M.A.-D.); [email protected] (A.C.-R.) 2 Facultad de Ciencias Biológicas y Agropecuarias Región Tuxpan, Universidad Veracruzana, Tuxpam 92870, Mexico; [email protected] 3 Laboratorio de Bioinformática y Bioestadística, Facultad de Ciencias Biológicas y Agropecuarias, Universidad Veracruzana, Córdoba 94945, Mexico; [email protected] 4 Red de Estudios Moleculares Avanzados, Instituto de Ecología, Xalapa 91073, Mexico; [email protected] 5 USDA-ARS Knipling-Bushland U.S. Veterinary Pest Genomics Center and Livestock Insects Research Laboratory, Kerrville, TX 78028, USA; [email protected] 6 USDA-ARS San Joaquin Valley Agricultural Sciences Center, Parlier, CA 93648, USA * Correspondence: [email protected]; Tel.: +52-(229)-9342075 Abstract: The tick Amblyomma mixtum is an ectoparasite of veterinary and public health importance because of its role as a vector of zoonotic pathogens. However, little is known about A. mixtum intraspecific variability and if morphological differentiation exists between populations across its geographic range. This study aimed to determine by electron microscopy the morphological vari- ability of A. mixtum populations in the state of Veracruz, which has a large livestock population Citation: Aguilar-Domínguez, M.; among states in Mexico. Forty male and 40 female A. mixtum collected from the 10 natural regions Romero-Salas, D.; Sánchez-Montes, S.; of Veracruz state were analyzed microscopically to accomplish main component analysis for each Serna-Lagunes, R.; Rosas-Saito, G.; sex. -

20210315092550 48603.Pdf
ORIGINAL RESEARCH published: 24 February 2021 doi: 10.3389/fimmu.2021.616343 Molecular Characterization and Immunological Evaluation of Truncated Babesia microti Rhoptry Neck Protein 2 as a Vaccine Candidate † † Yu chun Cai 1,2,3 , Chun li Yang 4 , Wei Hu 1,2,3,5, Peng Song 1,2,3, Bin Xu 1,2,3, Yan Lu 1,2,3, Lin Ai 1,2,3, Yan hong Chu 1,2,3, Mu xin Chen 1,2,3, Jia xu Chen 1,2,3* and Shao hong Chen1,2,3* 1 National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention, Shanghai, China, 2 Laboratory Edited by: of Parasite and Vector Biology, Ministry of Public Health, Shanghai, China, 3 WHO Collaborating Centre for Tropical Diseases, Wanderley De Souza, National Center for International Research on Tropical Diseases, Ministry of Science and Technology, Shanghai, China, Federal University of Rio de Janeiro, 4 Department of Clinical Research, The 903rd Hospital of PLA, Hangzhou, China, 5 School of Life Sciences, Fudan University, Brazil Shanghai, China Reviewed by: Junlong Zhao, Huazhong Agricultural University, Babesia microti is a protozoan that infects red blood cells. Babesiosis is becoming a new China global threat impacting human health. Rhoptry neck proteins (RONs) are proteins located Lan He, at the neck of the rhoptry and studies indicate that these proteins play an important role in Huazhong Agricultural University, China the process of red blood cell invasion. In the present study, we report on the bioinformatic *Correspondence: analysis, cloning, and recombinant gene expression of two truncated rhoptry neck Jia xu Chen proteins 2 (BmRON2), as well as their potential for incorporation in a candidate vaccine [email protected] fl Shao hong Chen for babesiosis. -
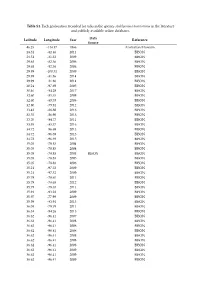
Table S1. Each Geolocation Recorded for Ticks in the Species Amblyomma Americanum in the Literature and Publicly Available Online Databases
Table S1. Each geolocation recorded for ticks in the species Amblyomma americanum in the literature and publicly available online databases. Data Latitude Longitude Year Reference Source 46.25 -114.17 1966 Australian Museum 28.31 -82.46 2011 BISON 28.51 -81.32 2009 BISON 29.68 -82.36 2006 BISON 29.68 -82.36 2006 BISON 29.99 -100.31 2009 BISON 29.99 -81.86 2014 BISON 29.99 -81.86 2014 BISON 30.24 -97.69 2005 BISON 30.46 -84.28 2017 BISON 32.60 -85.35 2008 BISON 32.60 -85.35 2008 BISON 32.80 -79.94 2012 BISON 33.42 -88.88 2016 BISON 33.55 -86.90 2013 BISON 33.70 -84.77 2011 BISON 33.95 -83.37 2016 BISON 34.72 -96.69 2013 BISON 34.72 -96.69 2013 BISON 34.72 -96.69 2015 BISON 35.05 -78.83 2004 BISON 35.05 -78.83 2004 BISON 35.05 -78.83 2004 BISON BISON 35.05 -78.83 2005 BISON 35.05 -78.83 2006 BISON 35.21 -97.32 2009 BISON 35.21 -97.32 2009 BISON 35.79 -78.65 2011 BISON 35.79 -78.65 2012 BISON 35.79 -78.65 2011 BISON 35.91 -93.22 2009 BISON 35.97 -77.99 2009 BISON 35.99 -83.94 2015 BISON 36.08 -79.79 2011 BISON 36.34 -94.26 2015 BISON 36.62 -96.41 2007 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2011 BISON 36.62 -96.41 2012 BISON 36.62 -96.41 2012 BISON 37.36 -77.05 2014 BISON 37.62 -84.87 2016 BISON 37.83 -78.28 2011 BISON 37.97 -85.70 2016 BISON 37.97 -85.70 2016 BISON 38.22 -75.31 2014 BISON 38.43 -88.43 -

Mechanisms Affecting the Acquisition, Persistence and Transmission Of
microorganisms Review Mechanisms Affecting the Acquisition, Persistence and Transmission of Francisella tularensis in Ticks Brenden G. Tully and Jason F. Huntley * Department of Medical Microbiology and Immunology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA; [email protected] * Correspondence: [email protected] Received: 29 September 2020; Accepted: 21 October 2020; Published: 23 October 2020 Abstract: Over 600,000 vector-borne disease cases were reported in the United States (U.S.) in the past 13 years, of which more than three-quarters were tick-borne diseases. Although Lyme disease accounts for the majority of tick-borne disease cases in the U.S., tularemia cases have been increasing over the past decade, with >220 cases reported yearly. However, when comparing Borrelia burgdorferi (causative agent of Lyme disease) and Francisella tularensis (causative agent of tularemia), the low infectious dose (<10 bacteria), high morbidity and mortality rates, and potential transmission of tularemia by multiple tick vectors have raised national concerns about future tularemia outbreaks. Despite these concerns, little is known about how F. tularensis is acquired by, persists in, or is transmitted by ticks. Moreover, the role of one or more tick vectors in transmitting F. tularensis to humans remains a major question. Finally, virtually no studies have examined how F. tularensis adapts to life in the tick (vs. the mammalian host), how tick endosymbionts affect F. tularensis infections, or whether other factors (e.g., tick immunity) impact the ability of F. tularensis to infect ticks. This review will assess our current understanding of each of these issues and will offer a framework for future studies, which could help us better understand tularemia and other tick-borne diseases.