Tree Species Profiles A8-1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tree Species Distribution Maps for Central Oregon
APPENDIX 7: TREE SPECIES DISTRIBUTION MAPS FOR CENTRAL OREGON A7-150 Appendix 7: Tree Species Distribution Maps Table A7-5. List of distribution maps for tree species of central Oregon. The species distribution maps are prefaced by four maps (pages A7-151 through A7-154) showing all locations surveyed in each of the four major data sources Map Page Forest Inventory and Analysis plot locations A7-151 Ecology core Dataset plot locations A7-152 Current Vegetation Survey plot locations A7-153 Burke Museum Herbarium and Oregon Flora Project sample locations A7-154 Scientific name Common name Symbol Abies amabilis Pacific silver fir ABAM A7-155 Abies grandis - Abies concolor Grand fir - white fir complex ABGR-ABCO A7-156 Abies lasiocarpa Subalpine fir ABLA A7-157 Abies procera - A. x shastensis Noble fir - Shasta red fir complex ABPR-ABSH A7-158 [magnifica x procera] Acer glabrum var. douglasii Douglas maple ACGLD4 A7-159 Alnus rubra Red alder ALRU2 A7-160 Calocedrus decurrens Incense-cedar CADE27 A7-161 Chrysolepis chrysophylla Golden chinquapin CHCH7 A7-162 Frangula purshiana Cascara FRPU7 A7-163 Juniperus occidentalis Western juniper JUOC A7-164 Larix occidentalis Western larch LAOC A7-165 Picea engelmannii Engelmann spruce PIEN A7-166 Pinus albicaulis Whitebark pine PIAL A7-167 Pinus contorta var. murrayana Sierra lodgepole pine PICOM A7-168 Pinus lambertiana Sugar pine PILA A7-169 Pinus monticola Western white pine PIMO3 A7-170 Pinus ponderosa Ponderosa pine PIPO A7-171 Populus balsamifera ssp. trichocarpa Black cottonwood POBAT A7-172 -
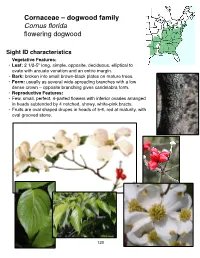
Cornaceae – Dogwood Family Cornus Florida Flowering Dogwood
Cornaceae – dogwood family Cornus florida flowering dogwood Sight ID characteristics Vegetative Features: • Leaf: 2 1/2-5" long, simple, opposite, deciduous, elliptical to ovate with arcuate venation and an entire margin. • Bark: broken into small brown-black plates on mature trees. • Form: usually as several wide-spreading branches with a low dense crown – opposite branching gives candelabra form. • Reproductive Features: • Few, small, perfect, 4-parted flowers with inferior ovaries arranged in heads subtended by 4 notched, showy, white-pink bracts. • Fruits are oval shaped drupes in heads of 5-6, red at maturity, with oval grooved stone. 123 NOTES AND SKETCHES 124 Cornaceae – dogwood family Cornus nuttallii Pacific dogwood Sight ID characteristics Vegetative Features: • Leaf: 2 1/2-4 1/2" long, simple, opposite, deciduous, ovate- elliptical with arcuate venation, margin may be sparsely toothed or entire. • Bark: dark and broken into small plates at maturity. • Form: straight trunk and narrow crown in forested conditions, many-trunked and bushy in open. • Reproductive Features: • Many yellowish-green, small, perfect, 4-parted flowers with inferior ovaries arranged in dense in heads, subtended by 4-7 showy white- pink, petal-like bracts - not notched at the apex. • Fruits are drupes in heads of 30-40, red at maturity and they have smooth stones. 125 NOTES AND SKETCHES 126 Cornaceae – dogwood family Cornus sericea red-osier dogwood Sight ID characteristics Vegetative Features: • Leaf: 2-4" long, simple, opposite, deciduous and somewhat narrow ovate-lanceolate with entire margin. • Twig: bright red, sometimes green splotched with red, white pith. • Bark: red to green with numerous lenticels; later developing larger cracks and splits and turning light brown. -

Western Juniper Woodlands of the Pacific Northwest
Western Juniper Woodlands (of the Pacific Northwest) Science Assessment October 6, 1994 Lee E. Eddleman Professor, Rangeland Resources Oregon State University Corvallis, Oregon Patricia M. Miller Assistant Professor Courtesy Rangeland Resources Oregon State University Corvallis, Oregon Richard F. Miller Professor, Rangeland Resources Eastern Oregon Agricultural Research Center Burns, Oregon Patricia L. Dysart Graduate Research Assistant Rangeland Resources Oregon State University Corvallis, Oregon TABLE OF CONTENTS Page EXECUTIVE SUMMARY ........................................... i WESTERN JUNIPER (Juniperus occidentalis Hook. ssp. occidentalis) WOODLANDS. ................................................. 1 Introduction ................................................ 1 Current Status.............................................. 2 Distribution of Western Juniper............................ 2 Holocene Changes in Western Juniper Woodlands ................. 4 Introduction ........................................... 4 Prehistoric Expansion of Juniper .......................... 4 Historic Expansion of Juniper ............................. 6 Conclusions .......................................... 9 Biology of Western Juniper.................................... 11 Physiological Ecology of Western Juniper and Associated Species ...................................... 17 Introduction ........................................... 17 Western Juniper — Patterns in Biomass Allocation............ 17 Western Juniper — Allocation Patterns of Carbon and -
1151CIRC.Pdf
CIRCULAR 153 MAY 1967 OBSERVATIONS on SPECIES of CYPRESS INDIGENOUS to the UNITED STATES Agricultural Experiment Station AUBURN UNIVERSIT Y E. V. Smith, Director Auburn, Alabama CONTENTS Page SPECIES AND VARIETIES OF CUPRESSUS STUDIED 4 GEOGRAPHIC DISTRIBUTION-- 4 CONE COLLECTION 5 Cupressus arizonica var. arizonica (Arizona Cypress) 7 Cupressus arizonica var. glabra (Smooth Arizona Cypress) 11 Cupressus guadalupensis (Tecate Cypress) 11 Cupressus arizonicavar. stephensonii (Cuyamaca Cypress) 11 Cupressus sargentii (Sargent Cypress) 12 Cupressus macrocarpa (Monterey Cypress) 12 Cupressus goveniana (Gowen Cypress) 12 Cupressus goveniana (Santa Cruz Cypress) 12 Cupressus goveniana var. pygmaca (Mendocino Cypress) 12 Cupressus bakeri (Siskiyou Cypress) 13 Cupressus bakeri (Modoc Cypress) 13 Cupressus macnabiana (McNab Cypress) 13 Cupressus arizonica var. nevadensis (Piute Cypress) 13 GENERAL COMMENTS ON GEOGRAPHIC VARIATION ---------- 13 COMMENTS ON STUDYING CYPRESSES 19 FIRST PRINTING 3M, MAY 1967 OBSERVATIONS on SPECIES of CYPRESS INDIGENOUS to the UNITED STATES CLAYTON E. POSEY* and JAMES F. GOGGANS Department of Forestry THERE HAS BEEN considerable interest in growing Cupressus (cypress) in the Southeast for several years. The Agricultural Experiment Station, Auburn University, was the first institution in the Southeast to initiate work on the cy- presses in 1937, and since that time many states have introduced Cupressus in hope of finding a species suitable for Christmas tree production. In most cases seed for trial plantings were obtained from commercial dealers without reference to seed source or form of parent tree. Many plantings yielded a high proportion of columnar-shaped trees not suitable for the Christmas tree market. It is probable that seed used in Alabama and other Southeastern States came from only a few trees of a given geo- graphic source. -

Botanical Memo
Appendix C Botanical Memo 10 May 2015 To Willow Creek Community Service District Copy to Patrick Kaspari, Senior Project Manager, GHD Inc. From Cara Scott, Botanist, GHD Inc. Tel 707.443.8326 Subject Special-Status Plant Species Survey and Mapping for Job no. 8410746.05 the Downtown Wastewater Development Project, Willow Creek, CA 1 Introduction On April 10 and May 8, 2015, special-status plant surveys and mapping were conducted for the proposed Downtown Wastewater Development Project in Willow Creek, Humboldt County, California . This survey attempted to identify all vascular plants within the project boundary and to document the presence of special-status plants. The purpose of these surveys was to map presence of special-status plant species and to document the approximate number of individuals and percent cover for each occurrence observed. The results will be used to reduce impacts associated with project construction and to avoid special-status plant populations 1.1 Location The unincorporated community of Willow Creek is located in Humboldt County approximately 45 miles northeast of Eureka, California as shown in Figure 1, Attachment 1. Willow Creek is situated along the Trinity River, which is part of the Klamath River Basin. The Willow Creek Community Services District (WCCSD or District) service area or district boundary is shown on Figure 2 and primarily consists of properties along State Highways 299 and 96. The Pacific Ocean is located approximately 26 miles to the west. The site corresponds to portions of Sections 32 and 33, Township 7 North, Range 5 East on the USGS 7.5 Minute Willow Creek and Salyer quadrangles. -

Likely to Have Habitat Within Iras That ALLOW Road
Item 3a - Sensitive Species National Master List By Region and Species Group Not likely to have habitat within IRAs Not likely to have Federal Likely to have habitat that DO NOT ALLOW habitat within IRAs Candidate within IRAs that DO Likely to have habitat road (re)construction that ALLOW road Forest Service Species Under NOT ALLOW road within IRAs that ALLOW but could be (re)construction but Species Scientific Name Common Name Species Group Region ESA (re)construction? road (re)construction? affected? could be affected? Bufo boreas boreas Boreal Western Toad Amphibian 1 No Yes Yes No No Plethodon vandykei idahoensis Coeur D'Alene Salamander Amphibian 1 No Yes Yes No No Rana pipiens Northern Leopard Frog Amphibian 1 No Yes Yes No No Accipiter gentilis Northern Goshawk Bird 1 No Yes Yes No No Ammodramus bairdii Baird's Sparrow Bird 1 No No Yes No No Anthus spragueii Sprague's Pipit Bird 1 No No Yes No No Centrocercus urophasianus Sage Grouse Bird 1 No Yes Yes No No Cygnus buccinator Trumpeter Swan Bird 1 No Yes Yes No No Falco peregrinus anatum American Peregrine Falcon Bird 1 No Yes Yes No No Gavia immer Common Loon Bird 1 No Yes Yes No No Histrionicus histrionicus Harlequin Duck Bird 1 No Yes Yes No No Lanius ludovicianus Loggerhead Shrike Bird 1 No Yes Yes No No Oreortyx pictus Mountain Quail Bird 1 No Yes Yes No No Otus flammeolus Flammulated Owl Bird 1 No Yes Yes No No Picoides albolarvatus White-Headed Woodpecker Bird 1 No Yes Yes No No Picoides arcticus Black-Backed Woodpecker Bird 1 No Yes Yes No No Speotyto cunicularia Burrowing -

Western Juniper Field Guide: Asking the Right Questions to Select Appropriate Management Actions
Western Juniper Field Guide: Asking the Right Questions to Select Appropriate Management Actions Circular 1321 U.S. Department of the Interior U.S. Geological Survey Cover: Photograph taken by Richard F. Miller. Western Juniper Field Guide: Asking the Right Questions to Select Appropriate Management Actions By R.F. Miller, Oregon State University, J.D. Bates, T.J. Svejcar, F.B. Pierson, U.S. Department of Agriculture, and L.E. Eddleman, Oregon State University This is contribution number 01 of the Sagebrush Steppe Treatment Evaluation Project (SageSTEP), supported by funds from the U.S. Joint Fire Science Program. Partial support for this guide was provided by U.S. Geological Survey Forest and Rangeland Ecosystem Science Center. Circular 1321 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia: 2007 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS--the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials con- tained within this report. Suggested citation: Miller, R.F., Bates, J.D., Svejcar, T.J., Pierson, F.B., and Eddleman, L.E., 2007, Western Juniper Field Guide: Asking the Right Questions to Select Appropriate Management Actions: U.S. -

Environmental Assessment
U.S. Department of the Interior Bureau of Land Management Environmental Assessment DOI-BLM-CA-N020-2018-0011-EA Timbered Crater ACEC/WSA Hazard Tree Removal Project May 2018 Craig Drake Applegate Field Office Manager 708 W. 12th Street Alturas, CA 96101 U.S. Department of the Interior Bureau of Land Management Applegate Field Office Phone: (530) 233-4666 Fax: (530) 233-5696 Contents 1. INTRODUCTION .................................................................................................................................................... 1 1.1 Background .............................................................................................................................................................. 1 1.2 Proposed Action Location ....................................................................................................................................... 2 1.3 Purpose and Need .................................................................................................................................................... 2 1.4 Scoping, Public Involvement, and Issues ................................................................................................................ 3 1.5 Plan Compliance and Tiering .................................................................................................................................. 3 Special Designations – Areas of Critical Environmental Concern: ............................................................................... 4 2. PROPOSED ACTION AND -

Oaks (Quercus Spp.): a Brief History
Publication WSFNR-20-25A April 2020 Oaks (Quercus spp.): A Brief History Dr. Kim D. Coder, Professor of Tree Biology & Health Care / University Hill Fellow University of Georgia Warnell School of Forestry & Natural Resources Quercus (oak) is the largest tree genus in temperate and sub-tropical areas of the Northern Hemisphere with an extensive distribution. (Denk et.al. 2010) Oaks are the most dominant trees of North America both in species number and biomass. (Hipp et.al. 2018) The three North America oak groups (white, red / black, and golden-cup) represent roughly 60% (~255) of the ~435 species within the Quercus genus worldwide. (Hipp et.al. 2018; McVay et.al. 2017a) Oak group development over time helped determine current species, and can suggest relationships which foster hybridization. The red / black and white oaks developed during a warm phase in global climate at high latitudes in what today is the boreal forest zone. From this northern location, both oak groups spread together southward across the continent splitting into a large eastern United States pathway, and much smaller western and far western paths. Both species groups spread into the eastern United States, then southward, and continued into Mexico and Central America as far as Columbia. (Hipp et.al. 2018) Today, Mexico is considered the world center of oak diversity. (Hipp et.al. 2018) Figure 1 shows genus, sub-genus and sections of Quercus (oak). History of Oak Species Groups Oaks developed under much different climates and environments than today. By examining how oaks developed and diversified into small, closely related groups, the native set of Georgia oak species can be better appreciated and understood in how they are related, share gene sets, or hybridize. -

Plant List As of 3/19/2008 Tanya Harvey T23S.R2E.S25, 36 *Non-Native
compiled by Bearbones Mountain Plant List as of 3/19/2008 Tanya Harvey T23S.R2E.S25, 36 *Non-native FERNS & ALLIES Taxaceae Quercus garryana Oregon white oak Dennstaediaceae Taxus brevifolia Pacific yew Pteridium aquilinum Garryaceae bracken fern TREES & SHRUBS: DICOTS Garrya fremontii Fremont’s silk tassel Dryopteridaceae Aceraceae Cystopteris fragilis Acer circinatum Grossulariaceae fragile fern vine maple Ribes roezlii var. cruentum shiny-leaved gooseberry, Sierra Polystichum imbricans Acer glabrum var. douglasii imbricate sword fern Douglas maple Ribes sanguineum red-flowering currant Polystichum munitum Acer macrophyllum sword fern big-leaf maple Hydrangeaceae Polypodiaceae Berberidaceae Philadelphus lewisii western mock orange Polypodium hesperium Berberis aquifolium western polypody shining Oregon grape Rhamnaceae Pteridiaceae Berberis nervosa Ceanothus prostratus Mahala mat Aspidotis densa Cascade Oregon grape indians’ dream Betulaceae Ceanothus velutinus snowbrush Cheilanthes gracillima Corylus cornuta var. californica lace fern hazelnut or filbert Rhamnus purshiana cascara Cryptogramma acrostichoides Caprifoliaceae parsley fern Lonicera ciliosa Rosaceae Pellaea brachyptera orange honeysuckle Amelanchier alnifolia western serviceberry Sierra cliffbrake Sambucus mexicana Selaginellaceae blue elderberry Holodiscus discolor oceanspray Selaginella scopulorum Symphoricarpos mollis Rocky Mountain selaginella creeping snowberry Oemleria cerasiformis indian plum Selaginella wallacei Celastraceae Prunus emarginata Wallace’s selaginella -

Botanischer Garten Der Universität Tübingen
Botanischer Garten der Universität Tübingen 1974 – 2008 2 System FRANZ OBERWINKLER Emeritus für Spezielle Botanik und Mykologie Ehemaliger Direktor des Botanischen Gartens 2016 2016 zur Erinnerung an LEONHART FUCHS (1501-1566), 450. Todesjahr 40 Jahre Alpenpflanzen-Lehrpfad am Iseler, Oberjoch, ab 1976 20 Jahre Förderkreis Botanischer Garten der Universität Tübingen, ab 1996 für alle, die im Garten gearbeitet und nachgedacht haben 2 Inhalt Vorwort ...................................................................................................................................... 8 Baupläne und Funktionen der Blüten ......................................................................................... 9 Hierarchie der Taxa .................................................................................................................. 13 Systeme der Bedecktsamer, Magnoliophytina ......................................................................... 15 Das System von ANTOINE-LAURENT DE JUSSIEU ................................................................. 16 Das System von AUGUST EICHLER ....................................................................................... 17 Das System von ADOLF ENGLER .......................................................................................... 19 Das System von ARMEN TAKHTAJAN ................................................................................... 21 Das System nach molekularen Phylogenien ........................................................................ 22 -

International Ecological Classification Standard
INTERNATIONAL ECOLOGICAL CLASSIFICATION STANDARD: TERRESTRIAL ECOLOGICAL CLASSIFICATIONS Groups and Macrogroups of Washington June 26, 2015 by NatureServe (modified by Washington Natural Heritage Program on January 16, 2016) 600 North Fairfax Drive, 7th Floor Arlington, VA 22203 2108 55th Street, Suite 220 Boulder, CO 80301 This subset of the International Ecological Classification Standard covers vegetation groups and macrogroups attributed to Washington. This classification has been developed in consultation with many individuals and agencies and incorporates information from a variety of publications and other classifications. Comments and suggestions regarding the contents of this subset should be directed to Mary J. Russo, Central Ecology Data Manager, NC <[email protected]> and Marion Reid, Senior Regional Ecologist, Boulder, CO <[email protected]>. Copyright © 2015 NatureServe, 4600 North Fairfax Drive, 7th floor Arlington, VA 22203, U.S.A. All Rights Reserved. Citations: The following citation should be used in any published materials which reference ecological system and/or International Vegetation Classification (IVC hierarchy) and association data: NatureServe. 2015. International Ecological Classification Standard: Terrestrial Ecological Classifications. NatureServe Central Databases. Arlington, VA. U.S.A. Data current as of 26 June 2015. Restrictions on Use: Permission to use, copy and distribute these data is hereby granted under the following conditions: 1. The above copyright notice must appear in all documents and reports; 2. Any use must be for informational purposes only and in no instance for commercial purposes; 3. Some data may be altered in format for analytical purposes, however the data should still be referenced using the citation above. Any rights not expressly granted herein are reserved by NatureServe.