An RNA-Binding Protein Family That Provides a Link Between Stem Cell Maintenance in Normal Development and Cancer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sample File CREDITS Lead Designer, Concept, Writing, and Art Direction Artwork Marc Altfuldisch the Artwork in This Handbook Is All Created by the Artists Below
Sample file CREDITS Lead Designer, Concept, Writing, and Art Direction Artwork Marc Altfuldisch The artwork in this handbook is all created by the artists below. A huge thanks goes out to them, for allowing me to include their illustrations herein. Balance and Flavor If you find their artwork intriguing, you should check out their galleries, which are linked Marc Altfuldisch below. Thomas Thorhave Baltzer George Cameron Alecyl Sandara David Moore alecyl.deviantart.com sandara.deviantart.com Bog Hag Page 3: Tree with Animals Editing Tsunami George Cameron Arturo Delgado Shaman with Animal Spirits madstalfos.deviantart.com Goi-Kashu Playtesters Jiki-Ketsu-Gaki Two-Tailed White Inari Adam Ford Con-Tinh Oriental Sea Life Alejandro Villalon Celestial Dragon and Human Bailey Kellenberger Carp Dragon and Human Girl Branden Weaver Dave Melvin davesrightmind.deviantart.com Celestial Dragon Bryan Butler Typhoon Dragon Kumo Derik Snell Byoki Spawn Nian Elvin Johson Kyoso Spawn Ashi no Oni George Cameron Sanru no Oni Haino no Oni Gianfranco Abbatemarco Akuma Kamu no Oni James “Dragon Lover” Hudson Byoki Ugulu no Oni Jason Gyorog Nikoma Kyoso John “Crit God” Wilantowicz Onikage Shikibu Jonathan Butler Phoenix Tsuburo Joseph Miller Great Wyrm Torn Kenneth Robinson Spirit Wolf Urigarimono Parker Doiron Taka-onna Yaoguai Pete McCue Wang-Liang Raymond Govero Robert “Wrayyth” Whitsell Harley Dela Cruz Shizen1102 Steven “Nook” Anderson denzelberg.deviantart.com shizen1102.deviantart.com Samantha Christine Bajang Garegosu Tre Stoterau Ancient Dokufu Void Serpent Victor Vega Dokufu Spiderling Kirin Vijay Dukkipati Goblin Rat Orochi Hellbeast Manananggal ... thank you all very much! Your assistance made this all possible! Hsing-sing Matriarch Hundun Hyekuhei Teo Tei Drone A Very Special Thanks to Kappa Tao Tei Regent Eleazzaar’s Detect Balance Werebadger Taowu Drone with Taowu Scouts SwordMeow’s JOTUNGARD Maho-Tsukai Magus Qiongqui Mamono .. -

American Motors ~I
American Motors ~I 1972 AMASPECIFICATIONS FORM . PassengerCar 1.-ANU FA C T URER CAR NA ME A ME RICAN M OT ORS CORPORATION • Grem li n • Ma t a dor • Jave li n • Horne t • Ambassa dor IAA I L I N G A OORESS MODEL. YEAR ISSUED : 14250 P l ymouth Road Det r o it, Mich igan 4823 2 1972 Se d:ember 2 1. 1971 At tn: Car L Chakmak ian REVISED (e ) P r oduc t Informat ion Dep t., 493 - 2557 (3 13) The information contained herein Is prepared, distributed by, and Is solely the responsi bility of the automobile manufacturing company to whose products it relates. Questions concerning these specifications should be directed to the manufacturer whose address Is shown above. This specification form was developed by automobile manufacturing companies under the auspices of the Automobile Manufacturers Association. AMA-40A-72 AMA Spec ificat ions Form-Pas senger Car TABLE OF CONTENTS BODY MODEL . ... .... .. ............... .. .... ......... ...... .... ...... , .... .. 1 CAR AND BODY DIMENSIONS ...... • .. .• ... , • •. ..• •. •.•.••. • •• •• .. .•• • . , ••. .. , , .•• 2, 3 POWER TEAMS .. ... .. ..... ... ........ .. ..... .. ..... ............... .... ........ 4 ENGINE .. ..................................... .. ..... .. .. .. ..... .... ... .. .. 5-9 EXHAUST SYSTEM ............................ ... ......• . ......................•... 9 FUEL SYSTEM ........................................ ... ...... ...... ...... .•..... 10 COOL ING SYSTEM ........................ .. ....... .... .. ....... ..... ........ 11 VEHICLE EMISSION CONTROL .... .. • ... -

The Gremlin: Transforming the Past's Failures 161 the Eagle Squadron, a Noted "Gremlinologist," Who Had Heard Many Such Tales and Was Beginning to Collect Them
The Gremlin: Transfor111ing the Past's Failures BRUCE A. ROSENBERG A MERICAN MOTORS CORPORATION entered the sub-compact field in 1970 with a sturdy little car it named the Gremlin. Its rear door-window (the hatch-back) descends so abruptly from the vehicle's roof that it gives it the appearance of having been cut off before it finished growing (or perhaps the way that Sir Yvain's horse was cut off at the hind quarter by the falling portcullis at the castle of Esclados the Red). The company it- self exploits the car's looks in television commercials which depict a snide and burly service-station attendant asking a pretty young driver about the "rest" of her car. But the ad concludes with the important message that though the car might have unorthodox looks it is economical to drive: the girl smugly and tauntingly hands her torrnentor a dollar bill for gas. What is most incongruous in all this, particularly for those people with clear memories of World War II, is the very name of the car, Gremlin. Historically this is the least likely name for a machine imaginable. But apparently not for the general public, and presumably not for the staff at American Motors which named the car several years ago. The company must have assumed, or conducted extensive field research which demonstrated, that car buyers would think of pixies, sprites, or other varieties of the "wee folk" when they heard the name Gremlin. The name conjures up an image of a small, compact creature (verymuch like the car), who is harmless, playful, and whimsical, perhaps something like the Celtic elf who sells the breakfast cereal, "Lucky Charms." When they first became known to Americans in 1942 (Gremlins had already made the acquaintance of the R.A.F.), they were the subject of several articles in leading magazines, and their diminutive stature was usually featured. -

Grapple Ball an Unholy Abomination of Wrestling and Basketball by Anon
Grapple Ball An unholy abomination of wrestling and basketball By anon A game where two 34 person teams attempt to score three points/hoops while wrestling and entertaining the crowd. Creating your Character: Pick a race, an affilliation, your gimmick, and determine skill modifiers/HP. There is 7 races to choose from: Dwarf Standing around 3'4", Dwarves are the tallest race. They weigh on average 46lb and have the most endurance. Dwarves are a heavyweight race and get +2 constitution. Might of the Mountain: Dwarves that stand still for one turn get +2 to the next grapple check they make. Dwarves move 4 hexes. Goblin Standing around 3'3", Goblins are the second tallest race. They weigh on average 50 lbs, making them the heaviest race, and have the most strength. Goblins are a heavyweight race and get +2 Strength. Fury of the Goblin King: Goblins enter RAGE mode at half health. Goblins move 4 hexes. Halfling Standing around 2'9", Halflings are the third tallest race. They weigh on average 34 lbs and are known for their luck and charm. Halflings are a medium race and get +2 favor. All Star: Masks give you favor no matter what. Halfings move 5 hexes. Kobold They stand around 2'7" and weigh on average 36 lbs. Kobolds are known for their keen senses. Kobolds are a medium race and get +1 strength and +1 constitution. Cockroaches Tenacity: When you would fall to 0hp you regain a quarter of your health, rounded down. Kobolds move 5 hexes. Gnome They stand around 2'1" and weigh on average 25 lbs. -

Graphic Novels Plan
Automatically Yours™ from Baker & Taylor Automatically Yours™ Graphic Novels Plan utomatically YoursTM from Baker & Taylor is a Specialized Collection Program that delivers the latest publications from popular authors right to Ayour door, automatically. Automatically Yours offers a variety of plans to meet your library’s needs including: Popular Adult Fiction Authors, B&T Kids, Spokenword Audio, Popular Book Clubs and Graphic Novels. Our Graphic Novels Plan delivers the latest publications to your library, automatically. Choose the novels that are right for your library and we’ll do the rest. No more placing separate orders, no worrying about title availability - they’ll arrive on time at your library. A new feature of the Automatically Yours program is updated lists of forthcoming titles available on our website, www.btol.com. Click on the Public Tab, then choose Automatic Shipments and Automatically Yours to view the current lists. Sign-up today! Simply fill out the enclosed listing by indicating the number of copies you require, complete the Account Information Form and return them both to Baker & Taylor. It’s that simple! Questions? Call us at 800.775.1200 x2315 or visit www.btol.com Automatically Yours™ from Baker & Taylor Sign up by Vendor/Character, Author or Illustrator. Fill in the quantity you need for each selection, fill out the Account Information Form and we’ll do the rest. It’s that simple! * Vendor/Character Listing: AIT-Planet Lar: ____ Hellboy - Adult Fantagraphics: ____ ALL Titles - Teen ____ Lone Wolf and Cub - Adult ____ ALL Hardcover Titles - Adult ____ O My Goddess - All Ages ____ ALL Paperback Titles - Adult Alternative Comics: ____ Predator - Teen ____ ALL Titles - Adult G.T. -

Saga of the Goblin Horde: Archetypes
By Richard "Zadmar" Woolcock Cover and interior page design by Lord Zsezse Works. Bugbear illustration by Storn Cook. Blue goblin illustration by Gary Dupuis. All other goblin illustrations by Rick Hershey. Saga of the Goblin Horde Archetypes version 6 © 2016 Richard Woolcock. Permission is granted to print this document for personal use only. Any electronic distribution of this document is strictly forbidden. Publisher's Choice Quality Stock Art © Rick Hershey / Fat Goblin Games (www.fatgoblingames.com). This game references the Savage Worlds game system, available from Pinnacle Entertainment Group at www.peginc.com. Savage Worlds and all associated logos and trademarks are copyrights of Pinnacle Entertainment Group. Used with permission. Pinnacle makes no representation or warranty as to the quality, viability, or suitability for purpose of this product. CCAANNIITTAAUURR CCRROOSSSSBBOOWWMMAANN DAEL DOGFOOT Goblins are an extremely prolific race, and occasionally prone to unusual mutations. You are a canitaur, a particularly rare type of goblin with the lower body of a canine, and you've learned to make the most of your mutation. Your natural gifts combined with your brutal attitude and tribal loyalty swiftly earned you the rank of gang boss. Your weapon of choice is the repeating crossbow, which allows you to rain death upon your foes while you use your superior speed to remain out of melee reach. However you are not shy about entering close combat when necessary, and wield your short handled hatchet with deadly efficiency. GAME STATISTICS -

Core Spell Tome 1
Core Spell Tome 1 CARD NAME TYPE # Copies CARD NAME TYPE # Copies Agony Enchantment 1 Leather Gloves Equipment 1 Animal Kinship Conjuration 1 Lightning Bolt Attack 1 Asyran Cleric Creature 1 Mage Staff Equipment 1 Banish Incantation 1 Mage Wand Equipment 1 Battle Fury Incantation 1 Maim Wings Enchantment 1 Bear Strength Enchantment 1 Mana Crystal Conjuration 1 Bitterwood Fox Creature 1 Mana Flower Conjuration 1 Block Enchantment 2 Mana Leech Creature 1 Blue Gremlin Creature 1 Marked for Death Enchantment 1 Bull Endurance Enchantment 1 Minor Heal Incantation 2 Call of the Wild Incantation 1 Mongoose Agility Enchantment 1 Charge Incantation 1 Moonglow Faerie Creature 1 Circle of Lightning Enchantment 1 Mountain Gorilla Creature 1 Darkfenne Bat Creature 1 Necropian Vampiress Creature 1 Darkpact Slayer Creature 1 Nullify Enchantment 2 Decoy Enchantment 1 Pacify Enchantment 1 Deflection Bracers Equipment 1 Perfect Strike Incantation 1 Dispel Incantation 2 Piercing Strike Incantation 1 Dissolve Incantation 1 Pillar of Light Attack 1 Divine Protection Enchantment 1 Poisoned Blood Enchantment 1 Dragonscale Hauberk Equipment 1 Purge Magic Incantation 1 Drain Life Incantation 1 Purify Incantation 1 Drain Power Incantation 1 Rajan's Fury Conjuration 1 Eagle Wings Enchantment 1 Regrowth Enchantment 1 Elemental Wand Equipment 1 Regrowth Belt Equipment 1 Enfeeble Enchantment 1 Retaliate Enchantment 1 Essence Drain Enchantment 1 Reverse Attack Enchantment 1 Evade Incantation 1 Reverse Magic Enchantment 1 Explode Incantation 1 Rhino Hide Enchantment 1 -

The Talepipe
The Talepipe March 2019 Fallbrook Vintage Car Club The Fallbrook Vintage Car Club is a group of members that share a common interest in the preservation and appreciation of vintage vehicles. We are dedicated to serving others through charitable events and activities that reflect positively on the Fallbrook community. A Region of the Antique Automobile Club of America About This Month’s Cover See the story about Bob Nixon elsewhere in this issue... The AMC Gremlin (also American Motors Gremlin) is an American subcompact automobile introduced in 1970, manufactured and marketed in a single, two-door body style in America (1970- 1978) by American Motors Corporation (AMC) — as well as in Mexico (1974-1978) by AMC’s Vehículos Automotores Mexicanos (VAM) subsidiary. Featuring a shortened Hornet platform and bodywork with a pronounced, almost vertical tail, the Gremlin was classified as an economy car by 1970’s U.S. standards. It competed with the Chevrolet Vega and Ford Pinto, as well as imported cars that included the Volkswagen Beetle and Toyota Corolla. The small domestic automaker marketed the Gremlin as “the first American-built import”. The Gremlin reached a total production of 671,475 over a single generation — and was superseded by a (thoroughly) restyled variant, the AMC Spirit. Designed to look either “cute or controversial - depending on one’s viewpoint ... for many, it seemed perfect for the free-thinking early 1970’s.” American Motors executives apparently felt confident enough to not worry that the Gremlin name might have negative connotations. Time magazine noted two definitions for gremlin: “Defined by Webster’s as ‘a small gnome held to be responsible for malfunction of equipment.’ American Motors’ definition: ‘a pal to its friends and an ogre to its enemies.’” The car’s cartoon-inspired mascot was marketed for product differentiation and was intended to be memorable to consumers. -

Gremlin Lore Gremlin Traits Water Gremlin
Gremlin Lore 15 years but they are fully sentient at the moment of their creation. Gremlins are strange little trixie menaces who are created by accident as a magical by Alignment. They are a chaotic product. Some gremlins manage to band bunch of little freaks but are not inherently together in and create magic rituals to make evil though their attempts at doing good are more. They often enjoy pranks instead of often misinterpreted. outright havoc; however, they are known to have a vengeful streak. They are also Size. Gremlins average height is extremely intelligent, but terrible at around 3 feet tall. Their average weight is 35 conveying any ideas more complex than pounds They count as small humanoids. what is achievable through cavemen talk. Gremlins vary in shape from type to type. Speed. Your base walking speed is Gremlins live throughout the world in 25 feet. random nooks and crannies that are related to their magical type. Jumbled Mind. Your mind is a garbled collection of thoughts that only you Faerûn Origins: can hope to truly understand. You have advantage saving throws against all effects The origins of gremlins in Faerûn is to try and magically charm, frighten, much like the origin of owlbears. One otherwise or control your mood. You also wizard, likely a Harpell, created a single cannot convey your ideas telepathically, nor magical life form. This first life form verbally very well often using the wrong escaped and multiplied. As the proto word or saying the opposite of what you gremlins encountered powerful magic mean. This does not affect your spell including elemental chaos cultists, mind casting. -

Theme Forces April 2021 Update
® APRIL 2021 Contents and Game Rules ©2001–2021 Privateer Press, Inc. All Rights Reserved. Privateer Press®, WARMACHINE®, Cephalyx, Convergence of Cyriss®, Convergence, Crucible Guard, Cryx®, Cygnar®, Khador®, Protectorate of Menoth®, Protectorate, Retribution of Scyrah®, Retribution, warcaster®, warjack®, HORDES®, Circle Orboros®, Circle, Grymkin, Grymkin: The Wicked Harvest, Legion of Everblight®, Legion, Skorne®, Trollbloods®, Trollblood, warbeast, and all associated logos and slogans are trademarks of Privateer Press, Inc. Permission is hereby granted to photocopy and retain electronic copies. Any such duplications shall be intended solely for noncommercial use and must maintain all copyrights, trademarks, or other notices contained therein or preserve all marks associated therewith. Privateer Press reserves the right to remove this permission or revise contents herein at any time for any reason. DARK LEGACY HEARTS OF DARKNESS As an ancient pact reaches its moment of reckoning, horrifying forces It is unlikely the invading Infernals would be as strong without the aid from beyond comprehension descend upon the world to collect. With of humans possessed of more selfish greed than survival instincts. As traitorous mortals as their momentary tools, Infernal Masters bring horrors move out from the shadows, people who have betrayed their their nightmares to battle for every soul they believe is their due. own kind hold the torches to light the way for evil. Perhaps they hope Though few would have suspected the terrible legacy that would follow to be spared by showing their allegiance and harvesting victims for that pact, many will pay for it as the Infernals move among them. their masters, but hope is a dying luxury now. -
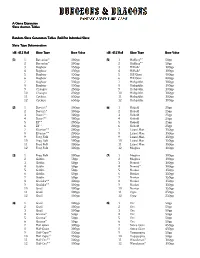
Game Expansion Slave Auction Tables
A Game Expansion Slave Auction Tables Random Slave Generation Tables (RollRoll Per Individual SlaveSlave) Slave Type Determination (d8dd8)/d128d8 d12 Rolld12 RollSlave TypeSlave Type Base ValueBase Value (d8d8d8d8)/d12d12 Rolld12 RollSlave TypeSlave Type Base ValueBase Value (111) 1 Berserker* 100gp (5555) 1 Halfling** 50gp 2 Berserker* 100gp 2 Halfling** 50gp 3 Bugbear 150gp 3 Hillfolk* 100gp 4 Bugbear 150gp 4 Hillfolk* 100gp 5 Bugbear 150gp 5 Hill Giant 400gp 6 Bugbear 150gp 6 Hill Giant 400gp 7 Bugbear 150gp 7 Hobgoblin 100gp 8 Bugbear 150gp 8 Hobgoblin 100gp 9 Chaugra 250gp 9 Hobgoblin 100gp 10 Chaugra 250gp 10 Hobgoblin 100gp 11 Cyclops 650gp 11 Hobgoblin 100gp 12 Cyclops 650gp 12 Hobgoblin 100gp ---------------------------------------------- ---------------------------------------------- (2222) 1 Dervish* 100gp (6666) 1 Kobold 25gp 2 Dervish* 100gp 2 Kobold 25gp 3 Dwarf** 100gp 3 Kobold 25gp 4 Dwarf** 100gp 4 Kobold 25gp 5 Elf** 200gp 5 Kobold 25gp 6 Elf** 200gp 6 Kobold 25gp 7 Elvarion** 200gp 7 Lizard Man 150gp 8 Elvarion** 200gp 8 Lizard Man 150gp 9 Frog Folk 100gp 9 Lizard Man 150gp 10 Frog Folk 100gp 10 Lizard Man 150gp 11 Frog Folk 100gp 11 Lizard Man 150gp 12 Frog Folk 100gp 12 Moghra 100gp ---------------------------------------------- ---------------------------------------------- (3333) 1 Frog Folk 100gp (7777) 1 Moghra 100gp 2 Goblin 50gp 2 Moghra 100gp 3 Goblin 50gp 3 Nomad* 100gp 4 Goblin 50gp 4 Nomad* 100gp 5 Goblin 50gp 5 Norker 150gp 6 Goblin 50gp 6 Norker 150gp 7 Goblin 50gp 7 Norker 150gp 8 Gnoldra** 100gp 8 Norker -

Recommended Reading List: All Ages Comics Are for Everyone, Both Kids and Adults, and These Comics Will Tickle the Fancy of Readers of All Ages
Recommended Reading List: All Ages Comics are for everyone, both kids and adults, and these comics will tickle the fancy of readers of all ages. Like Harry Potter, Calvin and Hobbes, or the Goonies? Can't get enough of Sabrina? Still catch yourself watching old cartoons or even new ones? Then go on and read about www.sequentialtart.com these kids and their fantastic adventures. Clan Apis Colonia by Jay Hosler by Jeff Nicholson Active Synapse (www.jayhosler.com) Colonia Press/AIT/Planet Lar (www.coloniapress.com) http://read.sequentialtart.com/?clanapis http://read.sequentialtart.com/?colonia You’ll enjoy this if you like: A Bug’s Life, Antz, The Lion King You’ll enjoy this if you like: Treasure Island, Kidnapped, Swiss Clan Apis tells the story of Nyuki, a honeybee, as she Family Robinson discovers life amongst her fellow honeybees and their A young boy and his guardians are plunged into another amazing social complexity. dimension where pirates still roam free, the American Revolution was never fought, and the United States is Herobear and the Kid Colonia, property of the British Empire. by Mike Kunkel Astonish Comics (www.theastonishfactory.com) Forty Winks http://read.sequentialtart.com/?herobear by Vince Sneed and John Peters You'll enjoy this if you like: The Wonder Years, Calvin and Hobbes Peregrine Entertainment (www.peregrine-entertain.com) Tyler is a young boy who inherits his grandfather's http://read.sequentialtart.com/?fortywinks broken pocket watch and a stuffed bear. He finds out that You’ll enjoy this if you like: Alice in Wonderland, Wizard of Oz there's more than meets the eye when the bear comes to 10-year-old Pandora Spocks travels to a magical world life – flapping red cape and all.