STATISTICAL ANALYSIS PLAN Title a Phase 2B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Detail Report
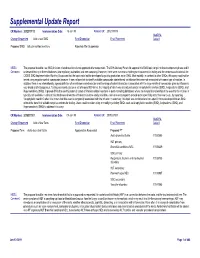
Supplemental Update Report CR Number: 2012319113 Implementation Date: 16-Jan-19 Related CR: 2012319113 MedDRA Change Requested Add a new SMQ Final Disposition Final Placement Code # Proposed SMQ Infusion related reactions Rejected After Suspension MSSO The proposal to add a new SMQ Infusion related reactions is not approved after suspension. The ICH Advisory Panel did approve this SMQ topic to go into the development phase and it Comment: underwent testing in three databases (two regulatory authorities and one company). However, there were numerous challenges encountered in testing and the consensus decision of the CIOMS SMQ Implementation Working Group was that the topic could not be developed to go into production as an SMQ. Most notably, in contrast to other SMQs, this query could not be tested using negative control compounds because it was not possible to identify suitable compounds administered via infusion that were not associated with some type of reaction. In addition, there is no internationally agreed definition of an infusion related reaction and the range of potential reactions associated with the large variety of compounds given by infusion is very broad and heterogenous. Testing was conducted on a set of around 500 terms, the majority of which was already included in Anaphylactic reaction (SMQ), Angioedema (SMQ), and Hypersensitivity (SMQ). It proved difficult to identify potential cases of infusion related reactions in post-marketing databases where the temporal relationship of the event to the infusion is typically not available. In clinical trial databases where this information is more easily available, users are encouraged to provide more specificity about the event, e.g., by reporting “Anaphylactic reaction” when it is known that this event is temporally associated with the infusion. -

COVID-19 Mrna Pfizer- Biontech Vaccine Analysis Print
COVID-19 mRNA Pfizer- BioNTech Vaccine Analysis Print All UK spontaneous reports received between 9/12/20 and 22/09/21 for mRNA Pfizer/BioNTech vaccine. A report of a suspected ADR to the Yellow Card scheme does not necessarily mean that it was caused by the vaccine, only that the reporter has a suspicion it may have. Underlying or previously undiagnosed illness unrelated to vaccination can also be factors in such reports. The relative number and nature of reports should therefore not be used to compare the safety of the different vaccines. All reports are kept under continual review in order to identify possible new risks. Report Run Date: 24-Sep-2021, Page 1 Case Series Drug Analysis Print Name: COVID-19 mRNA Pfizer- BioNTech vaccine analysis print Report Run Date: 24-Sep-2021 Data Lock Date: 22-Sep-2021 18:30:09 MedDRA Version: MedDRA 24.0 Reaction Name Total Fatal Blood disorders Anaemia deficiencies Anaemia folate deficiency 1 0 Anaemia vitamin B12 deficiency 2 0 Deficiency anaemia 1 0 Iron deficiency anaemia 6 0 Anaemias NEC Anaemia 97 0 Anaemia macrocytic 1 0 Anaemia megaloblastic 1 0 Autoimmune anaemia 2 0 Blood loss anaemia 1 0 Microcytic anaemia 1 0 Anaemias haemolytic NEC Coombs negative haemolytic anaemia 1 0 Haemolytic anaemia 6 0 Anaemias haemolytic immune Autoimmune haemolytic anaemia 9 0 Anaemias haemolytic mechanical factor Microangiopathic haemolytic anaemia 1 0 Bleeding tendencies Haemorrhagic diathesis 1 0 Increased tendency to bruise 35 0 Spontaneous haematoma 2 0 Coagulation factor deficiencies Acquired haemophilia -

Faculty Meeting August 9Th, 2011
Review of Systems is a process that includes a review of body systems. It is carried out through a series of questions regarding signs and symptoms. The Review of Systems (ROS) includes information about the following 14 systems. Constitutional: description of general appearance; growth and development, recent weight loss/gain, malaise, chills weakness, fatigue, fever, vital signs, head circumference for a baby, appetite, sleep habits, insomnia, night sweats. Integumentary: (skin and/or breast) rashes, color, sores, dryness, itching, flaking, dandruff, lumps, moles, color change, changes in hair or nails, sweating, hives, bruising, scratches, scars, swelling., acne. Eyes: vision, no change in vision, glasses or contact lenses, last eye exam, eye pain, “eye” redness, excessive tearing, double vision, blurred vision, spots, specks, flashing lights, photophobia, glaucoma, cataracts. Ears, Nose, Mouth/ Throat Ears: hearing loss, tinnitus, vertigo, earaches, ear infections, ear discharges; if hearing is decreased, use of hearing aids. Nose and sinuses: frequent colds, stuffiness’, discharge drainage, nasal itching, hay fever, nosebleeds sinusitis, sinus trouble, sinus pressure, nasal congestion, nasal discharge, nasal infection Mouth/Throat condition of teeth and gums bleeding gums dentures, (how they fit) last dental exam, dry mouth, frequent sore throats, difficulty swallowing, no posterior pharynx pain, hoarseness, sores/ulcers, hoarseness, pyorrhea. Respiratory: cough, sputum, (color, quantity) shortness of breath, pleuritic chest pain, wheezing, asthma, bronchitis, TB, emphysema, pneumonia, hemoptysis, CXR. Cardiovascular: heart trouble; high blood pressure; CV hypertension, heart murmurs, chest pain/ pressure palpitations, dyspnea, orthopnea,, rheumatic fever, paroxysmal nocturnal dyspnea, edema; past EKG or other heart tests. Peripheral Vascular; intermittent claudication, leg cramps, varicose veins, past clots in the vein, syncope, edema. -

Ekbom Syndrome: a Delusional Condition of “Bugs in the Skin”
Curr Psychiatry Rep DOI 10.1007/s11920-011-0188-0 Ekbom Syndrome: A Delusional Condition of “Bugs in the Skin” Nancy C. Hinkle # Springer Science+Business Media, LLC (outside the USA) 2011 Abstract Entomologists estimate that more than 100,000 included dermatophobia, delusions of infestation, and Americans suffer from “invisible bug” infestations, a parasitophobic neurodermatitis [2••]. Despite initial publi- condition known clinically as Ekbom syndrome (ES), cations referring to the condition as acarophobia (fear of although the psychiatric literature dubs the condition “rare.” mites), ES is not a phobia, as the individual is not afraid of This illustrates the reluctance of ES patients to seek mental insects but rather convinced that they are infesting his or health care, as they are convinced that their problem is her body [3, 4]. This paper deals with primary ES, not the bugs. In addition to suffering from the delusion that bugs form secondary to underlying psychological or physiologic are attacking their bodies, ES patients also experience conditions such as drug reaction or polypharmacy [5–8]. visual and tactile hallucinations that they see and feel the While Morgellons (“the fiber disease”) is likely a compo- bugs. ES patients exhibit a consistent complex of attributes nent on the same delusional spectrum, because it does not and behaviors that can adversely affect their lives. have entomologic connotations, it is not included in this discussion of ES [9, 10]. Keywords Parasitization . Parasitosis . Dermatozoenwahn . Valuable reviews of ES include those by Ekbom [1](1938), Invisible bugs . Ekbom syndrome . Bird mites . Infestation . Lyell [11] (1983), Trabert [12] (1995), and Bak et al. -

Chronic Pain:Useful Terms Personal Injury
Chronic pain:useful terms Personal Injury Analgesic - (also known as a painkiller) is any member of the group of drugs used to relieve pain. Epidural - Epidurals are given for the relief of pain. A cocktail of drugs containing a corticosteriod and a local anaesthetic is injected into the epidural space, between the bone and the membrane that encloses the spinal cord. Fusion - Surgical procedure designed to abolish movement across a joint. Usually involves bone grafting and sometimes metal fixation. Gout - Gout is a type of arthritis that causes sudden and extremely painful inflammatory attacks in the joints – most commonly the big toe, ankles and knees but any other joint too. Guanethidine Blocks – Tourniquet is applied to the limb and guanethidine is injected into a vein to temporarily treat Complex Regional Pain Syndrome symptoms. Hydrotherapy - formerly called hydropathy involves the use of water for pain-relief and treating illness. Hyperalgesia - The perception of a painful stimulus as more painful than normal. Instability - A term used to describe an abnormal increase in the movement of one vertebrae to another. Local Anaesthetic Blocks – Local anaesthetic is injected around the sympathetic nerves from a temporary sympathetic block to treat Complex Regional Pain Syndrome. MRI Scan - Magnetic Resonance Imaging involves a highly technical scanner that uses magnetic fields and computer technology to generate images of the internal anatomy of the body, including discs and nerve roots. It is a painless procedure, although like CT scans, people with claustrophobia may find it difficult. Myelography - A water-soluble, radio-opaque dye is injected into the cerebro-spinal fluid. -

Prognosis of a Case with Paresthesia Associated with Prolonged Touching of an Endodontic Paste to the Inferior Alveolar Nerve
J Clin Exp Dent. 2011;3(Suppl1):e377-81. Inferior alveolar nerve paresthesia. Journal section: Oral Surgery doi:10.4317/jced.3.e377 Publication Types: Case Report Prognosis of a case with paresthesia associated with prolonged touching of an endodontic paste to the inferior alveolar nerve M. Cemil Buyukkurt 1, Hakan Arslan 2, H. Sinan Topcuoglu 2, M. Melih Omezli 3 1 PhD, DDS. Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Sifa University, İzmir, Turkey 2 DDS. Department of Endodontic, Faculty of Dentistry, Ataturk University, Erzurum, Turkey 3 DDS. Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Ataturk University, Erzurum, Turkey Correspondence: Department of Endodontic, Faculty of Dentistry, Ataturk University, Erzurum, 25240, Turkey E-mail address: [email protected] Received: 10/02/2011 Accepted: 08/05/2011 Buyukkurt MC, Arslan H, Topcuoglu HS, Omezli MM. Prognosis of a case with paresthesia associated with prolonged touching of an endodontic paste to the inferior alveolar nerve. J Clin Exp Dent. 2011;3(Suppl1):e377- 81. http://www.medicinaoral.com/odo/volumenes/v3iSuppl1/jcedv3iSu- ppl1p377.pdf Article Number: 50506 http://www.medicinaoral.com/odo/indice.htm © Medicina Oral S. L. C.I.F. B 96689336 - eISSN: 1989-5488 eMail: [email protected] Abstract Paresthesia is described as an abnormal sensation, such as burning, pricking, tickling, tingling, formication or num- bness. Several conditions can cause paresthesia. This article presents a case of paresthesia caused by the extrusion of endodontic paste (Endomethasone®) into the mandibular canal. The clinical manifestations comprised the numb- ness on the right side of the mandible and right lower lip, appearing after endodontic treatment. -

Lyme Symptoms Check Sheet This List Is Not a Diagnosis, but Is Meant To
Lyme Symptoms Check sheet This list is not a diagnosis, but is meant to help you realize if Lyme Disease could be affecting you. Please remember that not all cases of Lyme Disease look the same, where some have over 100 symptoms, some have only two symptoms and both still test positive. Exposure: Have you had exposure to ticks? Were you ill after being bitten by a tick? Did you develop a target rash, or any other rash after being bitten by a tick? Do you have family members with Lyme Disease? Have you had sexual contact with someone with Lyme Disease? Have you been exposed to outdoor environment with brush, wild grasses, wild steams, golf courses, or woods in excess of 10 minutes in any location? Do you have pets who have been exposed to outdoor environment with brush, wild grasses, wild streams, golf courses, or woods in excess of 10 minutes in any location? Do you experience discomfort within two minutes of being in a musty or moldy location? Symptoms: Check all that apply Weight loss or gain in excess of 20 lbs in a short period of time Presence of any rash Changes in skin texture Slow wound healing Feeling worse or better after antibiotic treatment Poor short term memory Dizziness Vertigo Chemical Sensitivities Presence of a neurological disorder with or without a diagnosis Presence of a psychiatric disorder Facial paralysis or Bell’s Palsy Personality changes Anxiety Depression Irritability Rage Addictions Any of the following: paranoia, dementia, schizophrenia, bipolar disorder, panic attacks, major depression, -

Cases of Acute Mercury Poisoning by Mercury Vapor Exposure During The
Do et al. Annals of Occupational and Environmental Medicine (2017) 29:19 DOI 10.1186/s40557-017-0184-x CASE REPORT Open Access Cases of acute mercury poisoning by mercury vapor exposure during the demolition of a fluorescent lamp factory Sang Yoon Do1, Chul Gab Lee1, Jae Yoon Kim1, Young Hoon Moon1, Min Sung Kim2, In Ho Bae2 and Han Soo Song1* Abstract Background: In 2015, workers dismantling a fluorescent lamp factory in Korea were affected by mercury poisoning from exposure to mercury vapor. Case presentation: Eighteen out of the 21 workers who participated in the demolition project presented with symptoms of poisoning and, of these, 10 had persistent symptoms even at 18 months after the initial exposure to mercury vapor. Early symptoms of 18 workers included a general skin rash, pruritus, myalgia, sleep disturbance, and cough and sputum production. Following alleviation of these initial symptoms, late symptoms, such as easy fatigue, insomnia, bad dreams, and anxiety disorder, began to manifest in 10 out of 18 patients. Seven workers underwent psychiatric care owing to sleep disturbance, anxiety disorder, and depression, and three workers underwent dermatologic treatment for hyperpigmentation, erythematous skin eruption, and chloracne-like skin lesions. Furthermore, three workers developed a coarse jerky movement, two had swan neck deformity of the fingers, and two received care at an anesthesiology clinic for paresthesia, such as burning sensation, cold sensation, and pain. Two workers underwent urologic treatment for dysfunction of the urologic system and impotence. However, symptomatic treatment did not result in satisfactory relief of these symptoms. Conclusion: Awareness of the perils of mercury and prevention of mercury exposure are critical for preventing health hazards caused by mercury vapor. -

Neuropsychiatric Lyme Borreliosis: an Overview with a Focus on a Specialty Psychiatrist's Clinical Practice
healthcare Review Neuropsychiatric Lyme Borreliosis: An Overview with a Focus on a Specialty Psychiatrist’s Clinical Practice Robert C. Bransfield ID Department of Psychiatry, Rutgers-Robert Wood Johnson Medical School, Piscataway, NJ 08854, USA; bransfi[email protected]; Tel.: +1-732-741-3263; Fax.: +1-732-741-5308 Received: 10 July 2018; Accepted: 23 August 2018; Published: 25 August 2018 Abstract: There is increasing evidence and recognition that Lyme borreliosis (LB) causes mental symptoms. This article draws from databases, search engines and clinical experience to review current information on LB. LB causes immune and metabolic effects that result in a gradually developing spectrum of neuropsychiatric symptoms, usually presenting with significant comorbidity which may include developmental disorders, autism spectrum disorders, schizoaffective disorders, bipolar disorder, depression, anxiety disorders (panic disorder, social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder, intrusive symptoms), eating disorders, decreased libido, sleep disorders, addiction, opioid addiction, cognitive impairments, dementia, seizure disorders, suicide, violence, anhedonia, depersonalization, dissociative episodes, derealization and other impairments. Screening assessment followed by a thorough history, comprehensive psychiatric clinical exam, review of systems, mental status exam, neurological exam and physical exam relevant to the patient’s complaints and findings with clinical judgment, pattern recognition and knowledgeable -

Morgellons by Mary Leitao, Who Had a Son Who’M Suffered from This Condition
To the Lyme inquiry Panel. About three years ago I got an itchy sore that would not heal, then suddenly the sores spread over the bottom half of my legs and I also had sores on my hands and arms. I felt fatigued and had mysterious pains shooting through my nerves and bones I went to a local doctor who had no idea what was going on. To make a long story shorter I got treated for staph with antibiotics, and have had to take antibiotics for kidney and urinary tract infections three times in the past three years and once for lung infection. My partner looked at my sores with a magnifying glass and saw strange fibers in my skin, so I researched and found I had all the symptoms of what has been named morgellons by Mary Leitao, Who had a son who’m suffered from this condition. As I could not work and was very ill, I did attempt to see expensive private doctors as well as spending about $200 a fortnight on natural antibiotics, so given my research I gave up on asking for anything else from any doctor than monitoring of my blood to make sure all my vitamin and other levels were good, I do not.blame them for suggesting I am delusional, I know however how much pain I have been through and it amazes me how a delusion can manifest real sore and ulcers that will not heal. This is the description doctors see on wikipedia Morgellons From Wikipedia, the free encyclopedia Morgellons disease Classification and external resources Specialty Psychiatry MeSH D055535 [edit on Wikidata] Morgellons (/mɔː(ɹ)ˈdʒɛlənz/), also called Morgellons disease or Morgellons syndrome, is a condition in which people have the delusional belief that they are infested with disease-causing agents described as things like insects, parasites, hairs or fibers, while in reality no such things are present.[1] People with the condition may exhibit a range of cutaneous symptoms such as crawling, biting, and stinging sensations (formication), unusual fibers in the skin, and persistent skin lesions (e.g., rashes or sores). -

Treatment of Diabetic Neuropathy with a Novel PAR1-Targeting Molecule
biomolecules Article Treatment of Diabetic Neuropathy with A Novel PAR1-Targeting Molecule Efrat Shavit-Stein 1,2,* , Shany Guly Gofrit 2 , Alexandra Gayster 2, Yotam Teldan 2, Ariel Ron 2, Eiman Abu Bandora 2, Valery Golderman 1,2,3, Orna Gera 2,4, Sagi Harnof 5, Joab Chapman 1,2,3,6 and Amir Dori 1,2,7 1 Department of Neurology, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel; [email protected] (V.G.); [email protected] (J.C.); [email protected] (A.D.) 2 Department of Neurology, The Chaim Sheba Medical Center, Ramat Gan 5266202, Israel; [email protected] (S.G.G.); [email protected] (A.G.); [email protected] (Y.T.); [email protected] (A.R.); [email protected] (E.A.B.); [email protected] (O.G.) 3 Department of Physiology and Pharmacology, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel 4 Department of Physical Therapy, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel 5 Department of Neurosurgery, Rabin Medical Center, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel; [email protected] 6 Robert and Martha Harden Chair in Mental and Neurological Diseases, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel 7 Talpiot Medical Leadership Program, The Chaim Sheba Medical Center, Ramat Gan 5266202, Israel * Correspondence: [email protected]; Tel.: +972-3-5304409 Received: 7 October 2020; Accepted: 10 November 2020; Published: 13 November 2020 Abstract: Diabetic peripheral neuropathy (DPN) is a disabling common complication of diabetes mellitus (DM). -

B Disorders of Autonomic Nervous System
Application of the International Classification of Diseases to Neurology \ Second Edition World Health Organization Geneva 1997 First edition 1987 Second edition 1997 Application of the international classification of diseases to neurology: ICD-NA- 2nd ed. 1. Neurology - classification 2. Nervous system diseases - classification I. Title: ICD-NA ISBN 92 4 154502 X (NLM Classification: WL 15) The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. ©World Health Organization 1997 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers' products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of