PRODUCT MONOGRAPH Practemra
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients
antibodies Review Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients Andrew T. Lucas 1,2,3,*, Ryan Robinson 3, Allison N. Schorzman 2, Joseph A. Piscitelli 1, Juan F. Razo 1 and William C. Zamboni 1,2,3 1 University of North Carolina (UNC), Eshelman School of Pharmacy, Chapel Hill, NC 27599, USA; [email protected] (J.A.P.); [email protected] (J.F.R.); [email protected] (W.C.Z.) 2 Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] 3 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-919-966-5242; Fax: +1-919-966-5863 Received: 30 November 2018; Accepted: 22 December 2018; Published: 1 January 2019 Abstract: The rapid advancement in the development of therapeutic proteins, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), has created a novel mechanism to selectively deliver highly potent cytotoxic agents in the treatment of cancer. These agents provide numerous benefits compared to traditional small molecule drugs, though their clinical use still requires optimization. The pharmacology of mAbs/ADCs is complex and because ADCs are comprised of multiple components, individual agent characteristics and patient variables can affect their disposition. To further improve the clinical use and rational development of these agents, it is imperative to comprehend the complex mechanisms employed by antibody-based agents in traversing numerous biological barriers and how agent/patient factors affect tumor delivery, toxicities, efficacy, and ultimately, biodistribution. -
Detail Report
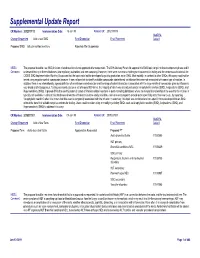
Supplemental Update Report CR Number: 2012319113 Implementation Date: 16-Jan-19 Related CR: 2012319113 MedDRA Change Requested Add a new SMQ Final Disposition Final Placement Code # Proposed SMQ Infusion related reactions Rejected After Suspension MSSO The proposal to add a new SMQ Infusion related reactions is not approved after suspension. The ICH Advisory Panel did approve this SMQ topic to go into the development phase and it Comment: underwent testing in three databases (two regulatory authorities and one company). However, there were numerous challenges encountered in testing and the consensus decision of the CIOMS SMQ Implementation Working Group was that the topic could not be developed to go into production as an SMQ. Most notably, in contrast to other SMQs, this query could not be tested using negative control compounds because it was not possible to identify suitable compounds administered via infusion that were not associated with some type of reaction. In addition, there is no internationally agreed definition of an infusion related reaction and the range of potential reactions associated with the large variety of compounds given by infusion is very broad and heterogenous. Testing was conducted on a set of around 500 terms, the majority of which was already included in Anaphylactic reaction (SMQ), Angioedema (SMQ), and Hypersensitivity (SMQ). It proved difficult to identify potential cases of infusion related reactions in post-marketing databases where the temporal relationship of the event to the infusion is typically not available. In clinical trial databases where this information is more easily available, users are encouraged to provide more specificity about the event, e.g., by reporting “Anaphylactic reaction” when it is known that this event is temporally associated with the infusion. -

Genentech Tocilizumab Letter of Authority June 24 2021
June 24, 2021 Hoffmann-La Roche, Ltd. C/O Genentech, Inc. Attention: Dhushy Thambipillai Regulatory Project Management 1 DNA Way, Bldg 45-1 South San Francisco, CA 94080 RE: Emergency Use Authorization 099 Dear Ms. Thambipillai: This letter is in response to Genentech, Inc.’s (Genentech) request that the Food and Drug Administration (FDA) issue an Emergency Use Authorization (EUA) for the emergency use of Actemra1 (tocilizumab) for the treatment of coronavirus disease 2019 (COVID-19) in certain hospitalized patients, as described in the Scope of Authorization (Section II) of this letter, pursuant to Section 564 of the Federal Food, Drug, and Cosmetic Act (the Act) (21 U.S.C. §360bbb-3). On February 4, 2020, pursuant to Section 564(b)(1)(C) of the Act, the Secretary of the Department of Health and Human Services (HHS) determined that there is a public health emergency that has a significant potential to affect national security or the health and security of United States citizens living abroad, and that involves the virus that causes coronavirus disease 2019 (COVID-19).2 On the basis of such determination, the Secretary of HHS on March 27, 2020, declared that circumstances exist justifying the authorization of emergency use of drugs and biological products during the COVID-19 pandemic, pursuant to Section 564 of the Act (21 3 U.S.C. 360bbb-3), subject to terms of any authorization issued under that section. Actemra is a recombinant humanized monoclonal antibody that selectively binds to both soluble and membrane-bound human IL-6 receptors (sIL-6R and mIL-6R) and subsequently inhibits IL- 6-mediated signaling through these receptors. -

Review Anti-Cytokine Biologic Treatment Beyond Anti-TNF in Behçet's Disease
Review Anti-cytokine biologic treatment beyond anti-TNF in Behçet’s disease A. Arida, P.P. Sfikakis First Department of Propedeutic Internal ABSTRACT and thrombotic complications (1-3). Medicine Laikon Hospital, Athens, Unmet therapeutic needs in Behçet’s Treatment varies according to type and University Medical School, Greece. disease have drawn recent attention to severity of disease manifestations. Cor- Aikaterini Arida, MD biological agents targeting cytokines ticosteroids, interferon-alpha and con- Petros P. Sfikakis, MD other than TNF. The anti-IL-17 anti- ventional immunosuppressive drugs, Please address correspondence to: body secukinumab and the anti-IL-2 such as azathioprine, cyclosporine-A, Petros P. Sfikakis, MD, receptor antibody daclizumab were not cyclophosphamide and methotrexate, First Department of Propedeutic superior to placebo for ocular Behçet’s and Internal Medicine, are used either alone or in combination Laikon Hospital, in randomised controlled trials, com- for vital organ involvement. During the Athens University Medical School, prising 118 and 17 patients, respec- last decade there has been increased use Ag Thoma, 17, tively. The anti-IL-1 agents anakinra of anti-TNF monoclonal antibodies in GR-11527 Athens, Greece. and canakinumab and the anti-IL-6 patients with BD who were refractory E-mail: [email protected] agent tocilizumab were given to iso- to conventional treatment or developed Received on June 7, 2014; accepted in lated refractory disease patients, who life-threatening complications (4, 5). revised form on September 17, 2014. were either anti-TNF naïve (n=9) or Anti-TNF treatment has been shown to Clin Exp Rheumatol 2014; 32 (Suppl. 84): experienced (n=18). -

Tocilizumab in Hospitalised Adults with Covid-19
This document has been approved for use at [ ] USE OF TOCILIZUMAB IN HOSPITALISED ADULTS WITH COVID-19 INFORMATION FOR PATIENTS, FAMILIES AND CARERS This information leaflet contains important information about the medicine called tocilizumab when used in the treatment of COVID-19. WHAT IS THE POTENTIAL BENEFIT OF It is important you provide your formal consent TOCILIZUMAB FOR COVID-19? before receiving tocilizumab. You can always change your mind about treatment with Tocilizumab belongs to a group of medicines called tocilizumab and withdraw consent at any time. monoclonal antibodies. Monoclonal antibodies are proteins, which specifically recognise and bind to unique proteins in the body to modify the way the WHAT SHOULD THE DOCTOR KNOW immune system works. Tocilizumab has effects on BEFORE TOCILIZUMAB IS USED IN the immune system that may be useful in helping COVID-19? to reduce the effects of severe COVID-19. The doctor should know about: Tocilizumab has been approved in Australia to treat any other conditions including HIV or AIDs, some immune conditions such as arthritis, cytokine tuberculosis, diverticulitis, stomach ulcers, release syndrome and giant cell arteritis. The brand diabetes, cancer, heart problems, raised name is Actemra®. blood pressure or any nerve disease such as Recent clinical trials studying the effectiveness of neuropathy tocilizumab in COVID-19 have been analysed by previous allergic reactions to any medicine Australia’s National COVID-19 Clinical Evidence all medicines including over-the-counter and Taskforce. The Taskforce makes recommendations complementary medicines e.g. vitamins, about when tocilizumab is most likely to be minerals, herbal or naturopathic medicines effective in the treatment of COVID-19. -

Tocilizumab in the Treatment of Severe and Refractory Parenchymal Neuro
TAB0010.1177/1759720X20971908Therapeutic Advances in Musculoskeletal DiseaseJ Liu, D Yan 971908research-article20202020 Therapeutic Advances in Musculoskeletal Disease Case Series Ther Adv Musculoskel Dis Tocilizumab in the treatment of severe and 2020, Vol. 12: 1–8 DOI:https://doi.org/10.1177/1759720X20971908 10.1177/ refractory parenchymal neuro-Behçet’s 1759720X20971908https://doi.org/10.1177/1759720X20971908 © The Author(s), 2020. Article reuse guidelines: syndrome: case series and literature review sagepub.com/journals- permissions Jinjing Liu* , Dong Yan*, Zhimian Wang, Yunjiao Yang, Shangzhu Zhang, Di Wu, Lingyi Peng, Zhichun Liu and Wenjie Zheng Abstract Correspondence to: Objectives: This study aimed to investigate the efficacy and safety of tocilizumab (TCZ) in Wenjie Zheng severe and refractory parenchymal neuro-Behçet’s syndrome (p-NBS). Department of Rheumatology and Clinical Methods: We retrospectively analyzed five patients with p-NBS treated with TCZ in our center Immunology, Peking Union between 2013 and 2020, and six cases from literature research with the index terms “neuro- Medical College Hospital, Chinese Academy of Behçet’s syndrome” and “tocilizumab” on PubMed NCBI. Medical Sciences & Peking Union Medical Results: A total of 11 patients with p-NBS were enrolled (5 males, 6 females), with a mean College, Key Laboratory of age of 34.5 ± 8.0 years at the onset. All the patients had parenchymal neurological lesions, Rheumatology and Clinical Rheumatology, Ministry six patients (54.5%) suffered from multiple lesions, and nine patients (81.8%) were disabled. of Education, National Clinical Research Center Before TCZ administration, all the patients had failed conventional therapy, eight patients for Dermatologic and (72.7%) received two or more immunosuppressants, and five patients showed insufficient Immunologic Diseases, No. -

Tocilizumab Preparation Risk Assessment
OUH General Risk Assessment Form Tocilizumab Preparation Risk Assessment It is recommended that all Trusts have in place a policy for the handling of mAbs. This should describe the responsibility of the Chief Pharmacist in defining the requirements needed for mAbs prepared outside the pharmacy, the resources available for handling of mAbs within the organisation, the mechanisms in place for the risk assessment of MAB handling and the clinical areas where mAbs may be used. This should be endorsed at Board level within the Trust and include a commitment to provide the resources necessary to allow the safe preparation of mAbs within the Trust in line with the guidance included within this document. It should also address the subject of the cost of provision of the resources required and how these are linked to the costs passed on to commissioners. Site JR Oxford University Hosptials NHS Trust Division CSS Directorate Covid wards/Pharmacy/ICU Department Pharmacy Location Exact N/A Date 21/04/2020 Assessors(s) 1 Clinical Divisional Pharmacist 2 Medicines Safety Pharmacist 3 Preparative Services Pharmacist 4 Senior Pharmacist Manager The Hazard or perceived risk Risks: 1. Risks of exposure to tocilizumab for staff handling the product due to the nature of or mode of action of the agent 2. Risks to patient receiving tocilizumab due to the potential for errors or contamination during the preparation of the product Cause: Need to determine the most appropriate area to prepare tocilizumab based on agreed MAB risk categories Affect: Staff could develop a reaction or adverse effect from tocilizumab. Patients may receive inaccurate product, with risk of microbiological contamination Impact: Minimise potential occupational exposure to staff and risk to patients receiving a dose Description (Identify who will be affected and how; include the context e.g. -

Successful Usage of Combination Biologic Therapy with Etanercept
Case Report Nov Tech Arthritis Bone Res Volume 2 Issue 4 - Febeuary 2018 Copyright © All rights are reserved by Yoon Kam Hon DOI: 10.19080/NTAB.2017.02.555592 Successful Usage of Combination Biologic Therapy with Etanercept and Actemra for the Treatment of TNF Inhibitor Failure Patients with Rheumatoid Arthritis: A Report of 2 Cases Yoon Kam Hon Department of Arthritis and Rheumatism Specialist Medical Centre, Singapore Submission: January 15, 2018; Published: February 02, 2018 *Corresponding author: Yoon Kam Hon, Consultant Physician And Rheumatologist, El Shaddai Arthritis and Rheumatism Specialist Medical Centre, Block 102 #01-258 Towner Road, Singapore 322102, Tel: +6581258239; Email: Abstract In the treatment of patients with rheumatoid arthritis with anti-tumour necrosis factor therapy, it is not uncommon for some patients to be unresponsive or relapse with treatment. We report the successful usage of combination biologic therapy with etanercept and tocilizumab in two factor and anti-interleukin 6 cytokine therapy in the treatment of rheumatoid arthritis. patients with moderately severe rheumatoid arthritis who failed etanercept therapy. This is the first report of combination anti-tumour necrosis Keywords: Rheumatoid arthritis; Combination biologic therapy; Etanercept; Tocilizumab Introduction therapy inadequate response (IR) RA patients in the RADIATE Rheumatoid arthritis (RA) is a complex chronic autoimmune trial. 50% of patients achieved ACR20 response at week 24, arthritis where many cytokines and chemokines including but only 20% achieved ACR50 response [5]. In the SUMMACTA Tumour Necrosis Factor (TNF), and Interleukin-6 (IL-6) play trial, it was shown that the intravenous (IV) form given at 8mg/ important roles in the pathophysiology of RA. -

COVID-19 Mrna Pfizer- Biontech Vaccine Analysis Print
COVID-19 mRNA Pfizer- BioNTech Vaccine Analysis Print All UK spontaneous reports received between 9/12/20 and 22/09/21 for mRNA Pfizer/BioNTech vaccine. A report of a suspected ADR to the Yellow Card scheme does not necessarily mean that it was caused by the vaccine, only that the reporter has a suspicion it may have. Underlying or previously undiagnosed illness unrelated to vaccination can also be factors in such reports. The relative number and nature of reports should therefore not be used to compare the safety of the different vaccines. All reports are kept under continual review in order to identify possible new risks. Report Run Date: 24-Sep-2021, Page 1 Case Series Drug Analysis Print Name: COVID-19 mRNA Pfizer- BioNTech vaccine analysis print Report Run Date: 24-Sep-2021 Data Lock Date: 22-Sep-2021 18:30:09 MedDRA Version: MedDRA 24.0 Reaction Name Total Fatal Blood disorders Anaemia deficiencies Anaemia folate deficiency 1 0 Anaemia vitamin B12 deficiency 2 0 Deficiency anaemia 1 0 Iron deficiency anaemia 6 0 Anaemias NEC Anaemia 97 0 Anaemia macrocytic 1 0 Anaemia megaloblastic 1 0 Autoimmune anaemia 2 0 Blood loss anaemia 1 0 Microcytic anaemia 1 0 Anaemias haemolytic NEC Coombs negative haemolytic anaemia 1 0 Haemolytic anaemia 6 0 Anaemias haemolytic immune Autoimmune haemolytic anaemia 9 0 Anaemias haemolytic mechanical factor Microangiopathic haemolytic anaemia 1 0 Bleeding tendencies Haemorrhagic diathesis 1 0 Increased tendency to bruise 35 0 Spontaneous haematoma 2 0 Coagulation factor deficiencies Acquired haemophilia -

Downloaded Here
Antibodies to Watch in a Pandemic Dr. Janice M. Reichert, Executive Director, The Antibody Society August 27, 2020 (updated slides) Agenda • US or EU approvals in 2020 • Granted as of late July 2020 • Anticipated by the end of 2020 • Overview of antibody-based COVID-19 interventions in development • Repurposed antibody-based therapeutics that treat symptoms • Newly developed anti-SARS-CoV-2 antibodies • Q&A 2 Number of first approvals for mAbs 10 12 14 16 18 20 Annual first approvals in either the US or EU or US the either in approvals first Annual 0 2 4 6 8 *Estimate based on the number actually approved and those in review as of July 15, with assumption of approval on the first c first the on of approval assumption 15, with as July of review in those and approved actually number the on based *Estimate Tables of approved mAbs and antibodies in review available at at mAbs ofand available in antibodies approved review Tables 1997 98 99 2000 01 02 03 Year of first US or EU approval or EU US of first Year 04 05 06 https://www.antibodysociety.org/resources/approved 07 08 09 10 11 12 13 14 15 Non-cancer Cancer 16 - antibodies/ 17 ycl 18 e. 19 2020* First approvals US or EU in 2020 • Teprotumumab (Tepezza): anti-IGF-1R mAb for thyroid eye disease • FDA approved on January 21 • Eptinezumab (Vyepti): anti-CGRP IgG1 for migraine prevention • FDA approved on February 21 • Isatuximab (Sarclisa): anti-CD38 IgG1 for multiple myeloma • FDA approved on March 2, also approved in the EU on June 2 • Sacituzumab govitecan (Trodelvy): anti-TROP-2 ADC for triple-neg. -

Faculty Meeting August 9Th, 2011
Review of Systems is a process that includes a review of body systems. It is carried out through a series of questions regarding signs and symptoms. The Review of Systems (ROS) includes information about the following 14 systems. Constitutional: description of general appearance; growth and development, recent weight loss/gain, malaise, chills weakness, fatigue, fever, vital signs, head circumference for a baby, appetite, sleep habits, insomnia, night sweats. Integumentary: (skin and/or breast) rashes, color, sores, dryness, itching, flaking, dandruff, lumps, moles, color change, changes in hair or nails, sweating, hives, bruising, scratches, scars, swelling., acne. Eyes: vision, no change in vision, glasses or contact lenses, last eye exam, eye pain, “eye” redness, excessive tearing, double vision, blurred vision, spots, specks, flashing lights, photophobia, glaucoma, cataracts. Ears, Nose, Mouth/ Throat Ears: hearing loss, tinnitus, vertigo, earaches, ear infections, ear discharges; if hearing is decreased, use of hearing aids. Nose and sinuses: frequent colds, stuffiness’, discharge drainage, nasal itching, hay fever, nosebleeds sinusitis, sinus trouble, sinus pressure, nasal congestion, nasal discharge, nasal infection Mouth/Throat condition of teeth and gums bleeding gums dentures, (how they fit) last dental exam, dry mouth, frequent sore throats, difficulty swallowing, no posterior pharynx pain, hoarseness, sores/ulcers, hoarseness, pyorrhea. Respiratory: cough, sputum, (color, quantity) shortness of breath, pleuritic chest pain, wheezing, asthma, bronchitis, TB, emphysema, pneumonia, hemoptysis, CXR. Cardiovascular: heart trouble; high blood pressure; CV hypertension, heart murmurs, chest pain/ pressure palpitations, dyspnea, orthopnea,, rheumatic fever, paroxysmal nocturnal dyspnea, edema; past EKG or other heart tests. Peripheral Vascular; intermittent claudication, leg cramps, varicose veins, past clots in the vein, syncope, edema. -

Asthma Agents
APPROVED PA Criteria Initial Approval Date: July 10, 2019 Revised Date: January 20, 2021 CRITERIA FOR PRIOR AUTHORIZATION Asthma Agents BILLING CODE TYPE For drug coverage and provider type information, see the KMAP Reference Codes webpage. MANUAL GUIDELINES Prior authorization will be required for all current and future dose forms available. All medication-specific criteria, including drug-specific indication, age, and dose for each agent is defined in Table 1 below. Benralizumab (Fasenra®) Dupilumab (Dupixent®) Mepolizumab (Nucala®) Omalizumab (Xolair®) Reslizumab (Cinqair®) GENERAL CRITERIA FOR INITIAL PRIOR AUTHORIZATION: (must meet all of the following) • Must be approved for the indication, age, and not exceed dosing limits listed in Table 1. • Must be prescribed by or in consultation with a pulmonologist, allergist, or immunologist.1,2 • For all agents listed, the preferred PDL drug, which treats the PA indication, is required unless the patient meets the non-preferred PDL PA criteria. • Must have experienced ≥ 2 exacerbations within the last 12 months despite meeting all of the following (exacerbation is defined as requiring the use of oral/systemic corticosteroids, urgent care/hospital admission, or intubation: o Patient adherence to two long-term controller medications, including a high-dose inhaled corticosteroid 1,2 (ICS) and a long-acting beta2-agonist (LABA) listed in Table 2. ▪ Combination ICS/LABA and ICS/LABA/LAMA products meet the requirement of two controller medications. o Patient must have had an adequate trial (at least 90 consecutive days) of a leukotriene modifier or a long-acting muscarinic antagonist (LAMA) as a third long-term controller medication listed in Table 2.