AGOSTONI-THESIS.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Harmful Algae 91 (2020) 101587
Harmful Algae 91 (2020) 101587 Contents lists available at ScienceDirect Harmful Algae journal homepage: www.elsevier.com/locate/hal Review Progress and promise of omics for predicting the impacts of climate change T on harmful algal blooms Gwenn M.M. Hennona,c,*, Sonya T. Dyhrmana,b,* a Lamont-Doherty Earth Observatory, Columbia University, Palisades, NY, United States b Department of Earth and Environmental Sciences, Columbia University, New York, NY, United States c College of Fisheries and Ocean Sciences University of Alaska Fairbanks Fairbanks, AK, United States ARTICLE INFO ABSTRACT Keywords: Climate change is predicted to increase the severity and prevalence of harmful algal blooms (HABs). In the past Genomics twenty years, omics techniques such as genomics, transcriptomics, proteomics and metabolomics have trans- Transcriptomics formed that data landscape of many fields including the study of HABs. Advances in technology have facilitated Proteomics the creation of many publicly available omics datasets that are complementary and shed new light on the Metabolomics mechanisms of HAB formation and toxin production. Genomics have been used to reveal differences in toxicity Climate change and nutritional requirements, while transcriptomics and proteomics have been used to explore HAB species Phytoplankton Harmful algae responses to environmental stressors, and metabolomics can reveal mechanisms of allelopathy and toxicity. In Cyanobacteria this review, we explore how omics data may be leveraged to improve predictions of how climate change will impact HAB dynamics. We also highlight important gaps in our knowledge of HAB prediction, which include swimming behaviors, microbial interactions and evolution that can be addressed by future studies with omics tools. Lastly, we discuss approaches to incorporate current omics datasets into predictive numerical models that may enhance HAB prediction in a changing world. -

Physiological Responses of Aureoumbra Lagunensis and Synechococcus Sp
Harmful Algae 6 (2007) 48–55 www.elsevier.com/locate/hal Physiological responses of Aureoumbra lagunensis and Synechococcus sp. to nitrogen addition in a mesocosm experiment Hudson R. DeYoe a,*, Edward J. Buskey b, Frank J. Jochem c a Department of Biology, University of Texas-Pan American, 1201 W. University Dr., Edinburg, TX 78541, United States b University of Texas Marine Science Institute, Port Aransas, TX 78373, United States c Florida International University, Marine Biology Program, North Miami, FL 33181, United States Received 7 March 2006; received in revised form 16 May 2006; accepted 6 June 2006 Abstract Aureoumbra lagunensis is the causative organism of the Texas brown tide and is notable because it dominated the Laguna Madre ecosystem from 1990 to 1997. This species is unusual because it has the highest known critical nitrogen to phosphorus ratio (N:P) for any microalgae ranging from 115 to 260, far higher than the 16N:1P Redfield ratio. Because of its high N:P ratio, Aureoumbra should be expected to respond to N additions that would not stimulate the growth of competitors having the Redfield ratio. To evaluate this prediction, a mesocosm experiment was performed in the Laguna Madre, a South Texas coastal lagoon, in which a mixed Aureoumbra–Synechococcus (a cyanobacterium) community was enclosed in 12 mesocosms and subjected to nitrogen addition (6 controls, 6 added ammonium) for 16 days. After day 4, added nitrogen did not significantly increase Aureoumbra specific growth rate but the alga retained dominance throughout the experiment (64–75% of total cell biovolume). In control mesocosms, Aureoumbra became less abundant during the first 4 days of the experiment but rebounded by the end of the experiment and was dominant over Synechococcus. -

HARMFUL ALGAL BLOOMS in COASTAL WATERS: Options for Prevention, Control and Mitigation
Science for Solutions A A Special Joint Report with the Decision Analysis Series No. 10 National Fish and Wildlife Foundation onald F. Boesch, Anderson, Rita A dra %: Shumway, . Tesf er, Terry E. February 1997 U.S. DEPARTMENT OF COMMERCE U.S. DEPARTMENT OF THE INTERIOR William M. Daley, Secretary Bruce Babbitt, Secretary The Decision Analysis Series has been established by NOAA's Coastal Ocean Program (COP) to present documents for coastal resource decision makers which contain analytical treatments of major issues or topics. The issues, topics, and principal investigators have been selected through an extensive peer review process. To learn more about the COP or the Decision Analysis Series, please write: NOAA Coastal Ocean Office 1315 East West Highway Silver Spring, MD 209 10 phone: 301-71 3-3338 fax: 30 1-7 13-4044 Cover photo: The upper portion of photo depicts a brown tide event in an inlet along the eastern end of Long Island, New York, during Summer 7986. The blue water is Block lsland Sound. Photo courtesy of L. Cosper. Science for Solutions NOAA COASTAL OCEAN PROGRAM Special Joint Report with the Decision Analysis Series No. 10 National Fish and WildlifeFoundation HARMFUL ALGAL BLOOMS IN COASTAL WATERS: Options for Prevention, Control and Mitigation Donald F. Boesch, Donald M. Anderson, Rita A. Horner Sandra E. Shumway, Patricia A. Tester, Terry E. Whitledge February 1997 National Oceanic and Atmospheric Administration National Fish and Wildlife Foundation D. James Baker, Under Secretary Amos S. Eno, Executive Director Coastal Ocean Office Donald Scavia, Director This ~ublicationshould be cited as: Boesch, Donald F. -

Effects of Harmful Algal Blooms Caused by Aureoumbra Lagunensis (Brown Tide) on Larval and Juvenile Life Stages of the Eastern Oyster (Crassostrea Virginica)
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2016 Effects of harmful algal blooms caused by Aureoumbra lagunensis (brown tide) on larval and juvenile life stages of the eastern oyster (Crassostrea virginica) Panagiota Makris University of Central Florida Part of the Biology Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Masters Thesis (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Makris, Panagiota, "Effects of harmful algal blooms caused by Aureoumbra lagunensis (brown tide) on larval and juvenile life stages of the eastern oyster (Crassostrea virginica)" (2016). Electronic Theses and Dissertations, 2004-2019. 5319. https://stars.library.ucf.edu/etd/5319 EFFECTS OF HARMFUL ALGAL BLOOMS CAUSED BY AUREOUMBRA LAGUNENSIS (BROWN TIDE) ON LARVAL AND JUVENILE LIFE STAGES OF THE EASTERN OYSTER (CRASSOSTREA VIRGINICA) by PANAYIOTA MAKRIS B.S. University of South Florida, 2012 A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in the Department of Biology in the College of Sciences at the University of Central Florida Orlando, Florida Spring Term 2016 Major Professor: Linda J. Walters ABSTRACT Harmful algal blooms caused by the marine microalga Aureoumbra lagunensis have been associated with negative impacts on marine fauna, both vertebrate and invertebrate. Within the Indian River Lagoon (IRL) estuary system along Florida’s east coast, blooms of A. -

Reassessing the Biodiversity of the Indian River Lagoon
Proceedings of Indian River Lagoon Symposium 2020 Reassessing the biodiversity of the Indian River Lagoon M. Dennis Hanisak(1) and Duane E. De Freese(2) (1)Harbor Branch Oceanographic Institute at Florida Atlantic University, 5600 U.S. 1 North, Fort Pierce, FL 34946 (2)IRL Council & Indian River Lagoon National Estuary Program, 1235 Main Street, Sebastian, FL 32958 Introduction The Indian River Lagoon (IRL), a unique, highly diverse, shallow-water estuary of national significance, stretches along 156 miles, ~40% of Florida’s east coast. The IRL’s annual economic value to Florida is estimated to be ~$7.6 billion (ECFRPC and TCRPC 2016). Urbanization, excessive freshwater releases, degradation of water quality, contaminant loading, loss of habitat, harmful algal blooms, decline of fisheries, and emerging diseases in marine mammals and other biota are increasingly important issues in the IRL (Sigua et al. 2000, Sime 2005, Reif et al. 2006, Taylor 2012, Phlips et al. 2015, Lapointe et al. 2015, Breininger et al. 2017), as they are throughout the world’s estuaries and coastal waters. In 1994, an Ad Hoc Committee established by the Indian River Lagoon National Estuary Program (IRLNEP) convened a conference on Biodiversity of the Indian River Lagoon in response to the lack of management planning on this topic. The goal of that two-day conference was to assemble and synthesize information on the status of biodiversity in the Lagoon. That synthesis contributed to management recommendations for inclusion in IRLNEP’s initial Comprehensive Conservation Management Plan (IRLNEP 1996). Proceedings of that conference were published as a dedicated issue (Bulletin of Marine Science 1995) that has served as an important reference for 25 years. -

Sublethal Effects of Texas Brown Tide on Streblospio Benedicti (Polychaeta) Larvae
Journal of Experimental Marine Biology and Ecology L 248 (2000) 121±129 www.elsevier.nl/locate/jembe Sublethal effects of Texas brown tide on Streblospio benedicti (Polychaeta) larvae Landon A. Ward, Paul A. Montagna* , Richard D. Kalke, Edward J. Buskey University of Texas at Austin, Marine Science Institute, 750 Channel View Drive, Port Aransas, TX 78373, USA Received 22 January 1998; received in revised form 13 January 2000; accepted 18 January 2000 Abstract The Texas brown tide bloom is noted for a concordant decline in benthic biomass and species diversity. However, the link between harmful effects induced by Texas brown tide and benthos has not been demonstrated. It has been proposed there may be a larval bottleneck, where larvae, but not adults, suffer adverse effects. This study was performed to test the effect of brown tide alga, Aureoumbra lagunensis, on mortality, growth and behavior of Streblospio benedicti larvae. Growth rates and swimming speeds, but not mortality rates, of polychaete larvae were reduced in cultures with brown tide relative to Isochrysis galbana, which is about the same size as brown tide. Results from this research indicate that brown tide does have harmful sublethal effects for one dominant species of meroplanktonic larvae, which could help explain reduced adult population size. 2000 Elsevier Science B.V. All rights reserved. Keywords: Brown tide; Polychaete larvae; Meroplankton; Growth; Mortality; Swimming speed; Turning rates 1. Introduction The Texas brown tide is a harmful algal bloom occurring in estuaries of the southern Texas coast, USA. The bloom was ®rst noted in January 1990 in Baf®n Bay and spread to Upper Laguna Madre in July 1990, where it persists as of this writing. -

Bivalve Feeding Responses to Microalgal Bloom Species in the Indian River Lagoon: the Potential for Top-Down Control
Estuaries and Coasts (2020) 43:1519–1532 https://doi.org/10.1007/s12237-020-00746-9 Bivalve Feeding Responses to Microalgal Bloom Species in the Indian River Lagoon: the Potential for Top-Down Control Eve Galimany1,2 & Jessica Lunt1,3 & Christopher J. Freeman1,4 & Jay Houk1 & Thomas Sauvage1,5 & Larissa Santos 1 & Jillian Lunt1 & Maria Kolmakova1 & Malcolm Mossop1 & Arthur Domingos1 & Edward J. Phlips6 & Valerie J. Paul1 Received: 6 January 2020 /Revised: 2 April 2020 /Accepted: 14 April 2020 / Published online: 23 April 2020 # The Author(s) 2020 Abstract In 2011, the Indian River Lagoon, a biodiverse estuary in eastern Florida (USA), experienced an intense microalgal bloom with disastrous ecological consequences. The bloom included a mix of microalgae with unresolved taxonomy and lasted for 7 months with a maximum concentration of 130 μg chlorophyll a L−1. In 2012, brown tide Aureoumbra lagunensis also bloomed in portions of this estuary, with reoccurrences in 2016 and 2018. To identify and understand the role of grazer pressure (top-down control) on bloom formation, we coupled DNA sequencing with bivalve feeding assays using three microalgae isolated from the 2011 bloom and maintained in culture. Feeding experiments were conducted on widely distributed bivalve species in the lagoon, including eastern oysters (Crassostrea virginica), hooked mussels (Ischadium recurvum), charru mussels (Mytella charruana), green mussels (Perna viridis), Atlantic rangia (Rangia cuneata), and hard clams (Mercenaria mercenaria), which were exposed to 3 × 104 cells mL−1 of five species of microalgae consisting of A. lagunensis and the three species clarified herein, the picocyanobacteria Crocosphaera sp. and ‘Synechococcus’ sp., and the picochlorophyte Picochlorum sp., as well as Nannochloropsis oculata used as a control. -
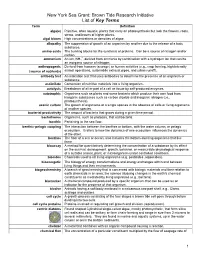
Key Terms For
New York Sea Grant: Brown Tide Research Initiative List of Key Terms Term Definition alga(e) Primitive, often aquatic, plants that carry on photosynthesis but lack the flowers, roots, stems, and leaves of higher plants. algal bloom High concentrations or densities of algae. allopathy The suppression of growth of an organism by another due to the release of a toxic substance. amino acids The building blocks for the synthesis of proteins. Can be a source of nitrogen and/or carbon. + ammonium An ion, NH4 , derived from ammonia by combination with a hydrogen ion that can be an inorganic source of nitrogen. anthropogenic Derived from humans (sewage) or human activities (e.g., crop farming, high intensity (source of nutrients) animal operations, automobile exhaust pipes, and urban runoff). antibody test An indication test that uses antibodies to determine the presence of an organism or substance. assimilate Conversion of nutritive materials into a living organism. autolysis Breakdown of all or part of a cell or tissue by self-produced enzymes. autotrophic Organisms such as plants and some bacteria which produce their own food from inorganic substances such as carbon dioxide and inorganic nitrogen (i.e., photosynthesis). axenic culture The growth of organisms of a single species in the absence of cells or living organisms of another species. bacterial productivity The amount of bacteria that grows during a given time period. bacterivores Organisms, such as protozoa, that eat bacteria. benthic Pertaining to the sea floor. benthic-pelagic coupling The interaction between the benthos or bottom, with the water column, or pelagic ecosystem. It refers to how the dynamics of one ecosystem influences the dynamics of the other. -

Does the Benthic Invertebrate Community Reflect Disturbances In
Proceedings of Indian River Lagoon Symposium 2020 Does the benthic invertebrate community reflect disturbances in the central Indian River Lagoon? Ann Wassick, Kelli Hunsucker, and Geoffrey Swain Center for Corrosion and Biofouling Control, Department of Ocean Engineering and Marine Sciences, Florida Institute of Technology, 150 W University Blvd, Melbourne, FL 32901 Abstract The Indian River Lagoon (IRL) is home to numerous species of plants and animals, including sessile benthic invertebrates. In addition to being an important component of the ecosystem, sessile benthic organisms may be of use to detect disturbances within the lagoon. This paper presents data from a long-term monitoring program of benthic invertebrate communities at a test site in the central lagoon since 2008. Over this time, these organisms endured the declining health of the IRL along with natural disturbances (i.e., cold snaps, algal blooms, tropical cyclones). Seasonal community composition was similar to what has previously been recorded, indicating that the health of the lagoon has not caused any long-term damage to benthic invertebrate community. However significant impacts were observed with barnacle and encrusting bryozoan cover as a result of various disturbances. Of the three types of disturbances investigated, the cold snap had no effect, the hurricane only had short-term impacts (weeks) and algal blooms had short- and long-term (months) impacts. Barnacle cover was severely reduced by algal blooms and at the same time the encrusting bryozoan cover increased. As algal blooms and anthropogenic disturbances become more frequent in the IRL, barnacles and encrusting bryozoans may be used as an indicator taxa for decreases in habitat quality. -

Ecological Aspects of Viral Infection and Lysis in the Harmful Brown Tide Alga Aureococcus Anophagefferens
AQUATIC MICROBIAL ECOLOGY Vol. 47: 25–36, 2007 Published April 3 Aquat Microb Ecol Ecological aspects of viral infection and lysis in the harmful brown tide alga Aureococcus anophagefferens Christopher J. Gobler1,*, O. Roger Anderson2, Mary Downes Gastrich2, Steven W. Wilhelm3 1Marine Sciences Research Center, Stony Brook University, Southampton, New York 11968, USA 2Lamont-Doherty Earth Observatory of Columbia University, 61 Route 9W, Palisades, New York 10964, USA 3Department of Microbiology, The University of Tennessee, Knoxville, Tennessee 37996, USA ABSTRACT: Although many bloom-forming phytoplankton are susceptible to viral lysis, the per- sistence of blooms of algal species that are susceptible to viral infection suggests that mechanisms prohibiting or minimizing viral infection and lysis are common. We describe the isolation of viruses capable of lysing the harmful brown tide pelagophyte Aureococcus anophagefferens and an investi- gation of factors which influence the susceptibility of A. anophagefferens cells to viral lysis. Nine strains of A. anophagefferens-specific viruses (AaV) were isolated from 2 New York estuaries which displayed 3-order of magnitude differences in ambient A. anophagefferens cell densities. The host range of AaV was species-specific and AaV was able to lyse 9 of 19 clonal A. anophagefferens cul- tures, suggesting that resistance to viral infection is common among these algal clones. Viral lysis of A. anophagefferens cultures was delayed at reduced light intensities, indicating that the low light conditions which prevail during blooms may reduce virally induced mortality of this alga. Some A. anophagefferens clones which were resistant to viral infection at 22°C were lysed by AaV at lower temperatures, suggesting that the induction of viral resistance at higher temperatures could allow the proliferation of blooms during summer months. -

Emaciated Black Drum Event
TEXAS PARKS AND WILDLIFE DEPARTMENT – COASTAL FISHERIES DIVISION Emaciated Black Drum Event Baffin Bay and the upper Laguna Madre Faye Grubbs, Art Morris, Alex Nunez, Zach Olsen, Jim Tolan 4/1/2013 Table of Contents Executive Summary ....................................................................................................................................... 2 Summary Report ........................................................................................................................................... 3 Introduction .............................................................................................................................................. 3 Upper Laguna Madre/Baffin Bay P. cromis ............................................................................................... 3 P. cromis Condition ................................................................................................................................... 4 Brown Tide Assessment ............................................................................................................................ 5 Status of ULM P. cromis ............................................................................................................................ 5 Status of P. cromis commercial fishery ..................................................................................................... 5 Salinity Trends .......................................................................................................................................... -
Supplemental Material
Supplemental Material Supplemental Figures a Others b 23 Methanobacteriales 25 Methanococcales 1800 1726 8 Thermoplasmatales 1600 36 Archaeoglobales 26 Methanomicrobiales 79 Methanocellales 1400 15 Methanosarcinales 1200 219 1000 Haloarchaea protein families f o r be 800 m Nu 58 Thermococcales 600 Others 451 400 70 Sulfolobales 200 81 4 Desulfurococcales 6 0 1 2 3 4 5 Thermoproteales Number of different gain events for the same protein family Others Supplemental Figure 1: Gain events for archaeal protein families calculated with Count’s birth-and-death model (NS dataset). (a) Reference tree of Archaeal genomes with the number of gain events calculated by Count for each archaeal group depicted in the respective node of the origin of the condensed phylum. (b) Number of different gains per protein family calculated by Count (no origins were calculated to occur at the root). Cathaya argyrophylla Pinus thunbergii Keteleeria davidiana Cedrus deodara Cryptomeria japonica Gnetum parvifoliua Welwitschia mirabilis Ephedra equisetina Cycas taitungensis Cheilanthes lindheimeri Adiantum capillusveneris Pteridium aquilinum subsp. aquilinum Alsophila spinulosa Angiopteris evecta Psilotum nudum Equisetum arvense Isoetes flaccida Selaginella moellendorffii Huperzia lucidula Anthoceros formosae Physcomitrella patens subsp. patens Syntrichia ruralis Marchantia polymorpha Staurastrum punctulatum Zygnema circumcarinatum Chara vulgaris Chaetosphaeridium globosum Mesostigma viride Chlorokybus atmophyticus Pseudendoclonium akinetum Oltmannsiellopsis viridis