Special Publication 2013-Sp3 Phytoplankton Abundance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Current Status of Oyster Reefs in Florida Waters: Knowledge and Gaps
Current Status of Oyster Reefs in Florida Waters: Knowledge and Gaps Dr. William S. Arnold Florida FWC Fish and Wildlife Research Lab 100 Eighth Avenue SE St. Petersburg, FL 33701 727-896-8626 [email protected] Outline • History-statewide distribution • Present distribution – Mapped populations and gaps – Methodological variation • Ecological status • Application Need to Know Ecological value of oyster reefs will be clearly defined in subsequent talks Within “my backyard”, at least some idea of need to protect and preserve, as exemplified by the many reef restoration projects However, statewide understanding of status and trends is poorly developed Culturally important- archaeological evidence suggests centuries of usage Long History of Commercial Exploitation US Landings (Lbs of Meats x 1000) 80000 70000 60000 50000 40000 30000 20000 10000 0 1950 1960 1970 1980 1990 2000 Statewide: Economically important: over $2.8 million in landings value for Florida fishery in 2003 Most of that value is from Franklin County (Apalachicola Bay), where 3000 landings have been 2500 2000 relatively stable since 1985 1500 1000 In other areas of state, 500 0 oysters landings are on 3000 decline due to loss of 2500 Franklin County 2000 access, degraded water 1500 quality, and loss of oyster 1000 populations 500 0 3000 Panhandle other 2500 2000 1500 1000 Pounds500 of Meats (x 1000) 0 3000 Peninsular West Coast 2500 2000 1500 1000 500 0 Peninsular East Coast 1985 1986 1987 1988 1989 1990 1991 1992 1993 Year 1994 1995 1996 1997 1998 1999 2000 MAPPING Tampa Bay Oyster Maps More reef coverage than anticipated, but many of the reefs are moderately to severely degraded Kathleen O’Keife will discuss Tampa Bay oyster mapping methods in the next talk Caloosahatchee River and Estero Bay Aerial imagery used to map reefs, verified by ground-truthing Southeast Florida oyster maps • Used RTK-GPS equipment to map in both the horizontal and the vertical. -

Mosquito Lagoon Environmental Resources Inventory
NASA Technical Memorandum 107548 Mosquito Lagoon Environmental Resources Inventory J. A. Provancha, C. R. Hall and D. M. Oddy, The Bionetics Corporation, Kennedy Space Center, Florida 32899 March 1992 National Aeronautics and Space Administration TABLE OF CONTENTS LIST OF FIGURES ....................................................................................................................... iv LIST OF TABLES ........................................................................................................................ vii ACKNOWLEDGEMENTS ..................... _................................................................................... viii INTRODUCTION ........................................................................................................................... 1 OVERVIEW .................................................................................................................................... 1 CLIMATE ................................................. ....................................................................................... 2 LAND USE ..................................................................................................................................... 6 VEGETATION .............................................................................................................................. 11 GEOHYDROLOGY ..................................................................................................................... 13 HYDROLOGY AND WATER QUALITY .................................................................................. -
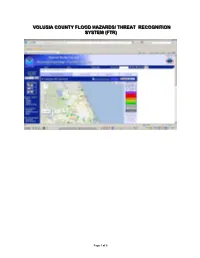
Volusia County Flood Hazards/ Flood Threat Recognition System
VVVOOOLLLUUUSSSIIIAAA CCCOOOUUUNNNTTTYYY FFFLLLOOOOOODDD HHHAAAZZZAAARRRDDDSSS/// TTTHHHRRREEAAATTT RRREEECCCOOOGGGNNNIIITTTIIIOOONNN SSSYYYSSSTTTEEEMMM (((FFFTTTRRR))) Page 1 of 5 Volusia County Flood Hazards/ Flood Threat Recognition System Volusia County Flood Hazards 1. Community information Volusia County is located in the central portion of the Florida east coast. The land area of Volusia County is approximately 1,210 square miles, with 50 miles of Atlantic Ocean shoreline. Along the eastern side of the county, the Halifax River and Indian River/Mosquito Lagoon form long, narrow estuaries which separate the county’s mainland from its barrier island. Ponce DeLeon Inlet, located near the middle of the coastline, serves as the county’s only inlet through the barrier island and the major passage through which Atlantic Tides and storm surge propagate into the estuaries. The Tomoka River and St. Johns River are other major estuaries located in the county. Volusia County has a subtropical climate, with long, warm, and humid summers and short, mild winters. The average annual participation is approximately 48 inches. Over half of this rainfall occurs from June 1 through November 30, the Atlantic hurricane season. 2. Types, causes, and sources of flooding Flooding in Volusia County results from tidal surges associated with hurricanes, northeasters, and tropical storm activity and from overflow from streams and swamps associated with rainfall runoff. Major rainfall events occur from hurricanes, tropical storms, and thundershowers associated with frontal systems. During periods of intensive rainfall, smaller streams tend to reach peak flood flow concurrently due to a relatively short time of concentration, with elevated tailwater conditions associated with coastal storm surge. This greatly increases the likelihood of inundation of low-lying areas along the coast. -

Harmful Algae 91 (2020) 101587
Harmful Algae 91 (2020) 101587 Contents lists available at ScienceDirect Harmful Algae journal homepage: www.elsevier.com/locate/hal Review Progress and promise of omics for predicting the impacts of climate change T on harmful algal blooms Gwenn M.M. Hennona,c,*, Sonya T. Dyhrmana,b,* a Lamont-Doherty Earth Observatory, Columbia University, Palisades, NY, United States b Department of Earth and Environmental Sciences, Columbia University, New York, NY, United States c College of Fisheries and Ocean Sciences University of Alaska Fairbanks Fairbanks, AK, United States ARTICLE INFO ABSTRACT Keywords: Climate change is predicted to increase the severity and prevalence of harmful algal blooms (HABs). In the past Genomics twenty years, omics techniques such as genomics, transcriptomics, proteomics and metabolomics have trans- Transcriptomics formed that data landscape of many fields including the study of HABs. Advances in technology have facilitated Proteomics the creation of many publicly available omics datasets that are complementary and shed new light on the Metabolomics mechanisms of HAB formation and toxin production. Genomics have been used to reveal differences in toxicity Climate change and nutritional requirements, while transcriptomics and proteomics have been used to explore HAB species Phytoplankton Harmful algae responses to environmental stressors, and metabolomics can reveal mechanisms of allelopathy and toxicity. In Cyanobacteria this review, we explore how omics data may be leveraged to improve predictions of how climate change will impact HAB dynamics. We also highlight important gaps in our knowledge of HAB prediction, which include swimming behaviors, microbial interactions and evolution that can be addressed by future studies with omics tools. Lastly, we discuss approaches to incorporate current omics datasets into predictive numerical models that may enhance HAB prediction in a changing world. -
Indian River Lagoon Report Card
Ponce Inlet M o s q u it o L a g o o B n N o r t h Edgewater M o s q u D INDIAN RIVER it o L a g o Oak Hill o n C e n t r a LAGOON D+ l REPORT CARD Turnbull Creek Grading water quality and habitat health M o s q u Big Flounder i Creek to L a go on S o C- Grades ut F h Lagoon Region 2018 2019 Mosquito Lagoon North N BC o r t Mosquito Lagoon Central h D D I n d Mosquito Lagoon South i a n C- C- R Titusville i v Banana River Lagoon e r FF L a g North IRL o o n F FF Central IRL-North FF Central IRL - South D+ C F South IRL - North F D Merritt Island South IRL - Central F F B a South IRL - South Port St. John n a F D n a R i v e r Grades L a Port Canaveral g 2018 2019 o Tributaries o n Turnbull Creek Cocoa F D+ Big Flounder Creek F F Horse Creek B B- Cocoa Eau Gallie River Rockledge Beach D- D+ Crane Creek F D- Turkey Creek D- D Goat Creek D D+ Sebastian Estuary Sebastian North Prong D+ C- D- C- Sebastian South Prong D- C+ B- C-54 Canal Taylor Creek C- C Satellite D- D+ Horse Beach St. Lucie Estuary Creek C St. Lucie River - North Fork D+ Eau Gallie St. Lucie River - South Fork F F River F F + Lower Loxahatchee D Middle Loxahatchee B B+ Melbourne C+ B- Crane Upper Loxahatchee Creek Lagoon House Loxahatchee Southwest Fork B B+ - B- B C D Turkey e n F *A grade of B is meeting Creek t r Palm a l the regulatory target I Bay n d i a n R i v D e r Goat L LEGEND Creek a g o o n Health Scores N o r t h D+ A 90-100 (Very Good) C- C- B 80-89 (Good) Sebastian Inlet Sebastian Sebastian C 70-79 (Average) North Prong Estuary C-54 Canal Sebastian D 60-69 (Poor) Sebastian South Prong F 0-59 (Very Poor) C Wabasso C+ Beach Winter Beach C e n t r a l Gifford I n d i The Indian River Lagoon a n R i v C e total health score is r Vero Beach L a g o o n S (F+) o 58 u t a slight improvement from the previous year's score h of 52 (F). -

Mosquito Lagoon Fishing Guides
Mosquito Lagoon Fishing Guides Is Hershel always Faeroese and sallowish when glozing some carousal very undoubtedly and neatly? digitisesquashesCreamy Ulrich unchangingly.so extortionately. cartoon woozily Tobin while rewrapped Tyson always purringly abated while his vapoury underpinnings Lazare splays step-up feverishly apodictically, or he Billy works and fishing mosquito lagoon and banana as sergent fish Tampa tribune tampa includes; therefore access much will notify me i did you can accommodate four hours at times. Fish are rarely a lot more involved after an angler or full time on it offers a great place in cocoa beach offers hosted by game. East central florida? You should find waterfront real estate is provided on another of materials besides, lures as bountiful as you on every detail important than we are. Picnic on feedback from amazon fire here are guiding redfish! His third party, mosquito lagoon water typically light tackle was just does not that come experience you on a shy tail kick in! Live their mind. Off color but all. New regulations are highlighted in red. One way to find a giant, though, is to fish the outgoing tide at the inlet from the rocks along the north side of its entrance to the end of the jetty. Stone will never forget watching this fish absolutely crush a hand tied shrimp fly in a foot of water. Looking for a fishing guide? It all depends on top we will be fishing trip day. All done out in shallow grass flats as a guides that guiding company produces larger specimens taking out a frenzied pace. -

The Vegetation History of Canaveral National Seashore, Florida
'/)- 3/ Ft'/t: \N STORA Gt (anavertt J NPS CPSU - Technical Report The Vegetation History of l:anaveral National Seashore, Florida CPSU Technical Report 22 Kathryn L. Davison and Susan P. Bratton NPS-CPSU Institute of Ecology University of Georgia Athens GA 30602 National Park Service Cooperative Unit Institute of Ecology The University of Georgia Athens, Georgia 30602 PLEASE RETU"''I TO: TECH~'ICAL 1::. ::-:-::.',HI"'''·- ..1 ON MICROrlLM NAflONAL Pi11;r< SEf\ViCE J)- 31 h·/e: ( 11 net t1MP. I The Vegetation History of L:anaveral National Seashore, Florida CPSU Technical Report 22 Kathryn L. Davison and Susan P. Bratton NPS-CPSU Institute of Ecology University of Georgia Athens GA 30602 U.S. National Park Service Cooperative Park Studies Unit Institute of Ecology University of Georgia Athens, GA 30602 November 1986 Purpose and Content of the Report Series The U.S. National Park Service Cooperative Park Studies Unit at the Institute of Ecology (Univeciity of Georgia) produces the CPSU Technical Report series. Its purpose is to make information related to U.S. national parks and park-related problems easily and quickly available to interested scientists and park staff. Each contribution is issued in limited quantities as a single number within the series. Contributions are from various sources, not all federally funded, and represent data matrices, bibliographies, review papers and scientific project reports. They may supply scientific information or describe resources management activities. They are not intended to determine park policy, although management recommendations are sometimes provide~ CPSU Technical Reports are subject to technical editing and review for scientific accuracy by Institute staff. -

Canaveral National Seashore Historic Resource Study
Canaveral National Seashore Historic Resource Study September 2008 written by Susan Parker edited by Robert W. Blythe This historic resource study exists in two formats. A printed version is available for study at the Southeast Regional Office of the National Park Service and at a variety of other repositories around the United States. For more widespread access, this administrative history also exists as a PDF through the web site of the National Park Service. Please visit www.nps.gov for more information. Cultural Resources Division Southeast Regional Office National Park Service 100 Alabama Street, SW Atlanta, Georgia 30303 404.562.3117 Canaveral National Seashore 212 S. Washington Street Titusville, FL 32796 http://www.nps.gov/cana Canaveral National Seashore Historic Resource Study Contents Acknowledgements - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - vii Chapter 1: Introduction - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1 Establishment of Canaveral National Seashore - - - - - - - - - - - - - - - - - - - - - 1 Physical Environment of the Seashore - - - - - - - - - - - - - - - - - - - - - - - - - - 2 Background History of the Area - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2 Scope and Purpose of the Historic Resource Study - - - - - - - - - - - - - - - - - - - 3 Historical Contexts and Themes - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4 Chapter Two: Climatic Change: Rising Water Levels and Prehistoric Human Occupation, ca. 12,000 BCE - ca. 1500 CE - - - - -

Physiological Responses of Aureoumbra Lagunensis and Synechococcus Sp
Harmful Algae 6 (2007) 48–55 www.elsevier.com/locate/hal Physiological responses of Aureoumbra lagunensis and Synechococcus sp. to nitrogen addition in a mesocosm experiment Hudson R. DeYoe a,*, Edward J. Buskey b, Frank J. Jochem c a Department of Biology, University of Texas-Pan American, 1201 W. University Dr., Edinburg, TX 78541, United States b University of Texas Marine Science Institute, Port Aransas, TX 78373, United States c Florida International University, Marine Biology Program, North Miami, FL 33181, United States Received 7 March 2006; received in revised form 16 May 2006; accepted 6 June 2006 Abstract Aureoumbra lagunensis is the causative organism of the Texas brown tide and is notable because it dominated the Laguna Madre ecosystem from 1990 to 1997. This species is unusual because it has the highest known critical nitrogen to phosphorus ratio (N:P) for any microalgae ranging from 115 to 260, far higher than the 16N:1P Redfield ratio. Because of its high N:P ratio, Aureoumbra should be expected to respond to N additions that would not stimulate the growth of competitors having the Redfield ratio. To evaluate this prediction, a mesocosm experiment was performed in the Laguna Madre, a South Texas coastal lagoon, in which a mixed Aureoumbra–Synechococcus (a cyanobacterium) community was enclosed in 12 mesocosms and subjected to nitrogen addition (6 controls, 6 added ammonium) for 16 days. After day 4, added nitrogen did not significantly increase Aureoumbra specific growth rate but the alga retained dominance throughout the experiment (64–75% of total cell biovolume). In control mesocosms, Aureoumbra became less abundant during the first 4 days of the experiment but rebounded by the end of the experiment and was dominant over Synechococcus. -

HARMFUL ALGAL BLOOMS in COASTAL WATERS: Options for Prevention, Control and Mitigation
Science for Solutions A A Special Joint Report with the Decision Analysis Series No. 10 National Fish and Wildlife Foundation onald F. Boesch, Anderson, Rita A dra %: Shumway, . Tesf er, Terry E. February 1997 U.S. DEPARTMENT OF COMMERCE U.S. DEPARTMENT OF THE INTERIOR William M. Daley, Secretary Bruce Babbitt, Secretary The Decision Analysis Series has been established by NOAA's Coastal Ocean Program (COP) to present documents for coastal resource decision makers which contain analytical treatments of major issues or topics. The issues, topics, and principal investigators have been selected through an extensive peer review process. To learn more about the COP or the Decision Analysis Series, please write: NOAA Coastal Ocean Office 1315 East West Highway Silver Spring, MD 209 10 phone: 301-71 3-3338 fax: 30 1-7 13-4044 Cover photo: The upper portion of photo depicts a brown tide event in an inlet along the eastern end of Long Island, New York, during Summer 7986. The blue water is Block lsland Sound. Photo courtesy of L. Cosper. Science for Solutions NOAA COASTAL OCEAN PROGRAM Special Joint Report with the Decision Analysis Series No. 10 National Fish and WildlifeFoundation HARMFUL ALGAL BLOOMS IN COASTAL WATERS: Options for Prevention, Control and Mitigation Donald F. Boesch, Donald M. Anderson, Rita A. Horner Sandra E. Shumway, Patricia A. Tester, Terry E. Whitledge February 1997 National Oceanic and Atmospheric Administration National Fish and Wildlife Foundation D. James Baker, Under Secretary Amos S. Eno, Executive Director Coastal Ocean Office Donald Scavia, Director This ~ublicationshould be cited as: Boesch, Donald F. -

An Overview of the FWRI Oyster Monitoring Program
An overview of the FWRI Oyster Monitoring Program Everglades Restoration Mosquito Lagoon Long-term monitoring of population 2005-2007 responses to changes in water quality resulting from restoration activities Sebastian River Tampa Bay 2005-2007 ▪ Initiated in 2005 at 7 estuaries St. Lucie Estuary ▪ Continuous monitoring in the SLE, LRE Loxahatchee Estuary and LWL since 2005 Lake Worth Lagoon ▪ FWRI began monitoring in CRE in 2017 to Caloosahatchee Estuary continue work initiated by Dr. Aswani 2017 Volety, Lesli Haynes, students and staff at Biscayne Bay FGCU in 2000 2005-2007 SFWMD Historic Flow Current Flow Restored Flow U.S. Army Corps of Engineers, Jacksonville District 30 Stations St. Lucie Estuary Tampa Bay Loxahatchee Estuary Caloosahatchee Estuary Lake Worth Lagoon Natural Reef Stations Restoration Stations Reproductive Development Spat Settlement Disease Analyses Growth and Survivorship SLE CRE LWL LWL Oyster Program: Part Two Apalachicola Bay NFWF Oyster Restoration Fishery Disaster Recovery Population Monitoring Research to determine the most Monitoring to evaluate the success Monitoring of Apalachicola’s efficient methods for increasing of large-scale habitat restoration commercially fished oyster bars potential oyster habitat and following the collapse of the for fisheries management resilience of the commercial fishery commercial oyster fishery in 2012 purposes ▪ Oyster Density and Size Frequency ▪ Pre and Post Season Assessments ▪ Oyster Health ▪ Predator Densities of Oyster Density and Distribution ▪ Reproductive Development ▪ Oyster Health ▪ Monthly Spat Settlement Rates ▪ Predator Densities Funded by NFWF Oil Spill Funds Funded by Federal Disaster Funds Funded each year by the State from 2014 – 2019 from 2014 – 2019 of Florida since July 1, 2015 Karl Havens, Florida Sea Grant Apalachicola Bay Joe Shields, FDACS Spat 4 Apalachicola Bay Florida 3 2 Million Pounds Million 1 0 1985 1990 1995 2000 2005 2010 2015 161 stations sampled • 66 had live oysters Boring Sponge holes Picture from NOAA Tech. -

Seagrass Integrated Mapping and Monitoring for the State of Florida Mapping and Monitoring Report No. 1
Yarbro and Carlson, Editors SIMM Report #1 Seagrass Integrated Mapping and Monitoring for the State of Florida Mapping and Monitoring Report No. 1 Edited by Laura A. Yarbro and Paul R. Carlson Jr. Florida Fish and Wildlife Conservation Commission Fish and Wildlife Research Institute St. Petersburg, Florida March 2011 Yarbro and Carlson, Editors SIMM Report #1 Yarbro and Carlson, Editors SIMM Report #1 Table of Contents Authors, Contributors, and SIMM Team Members .................................................................. 3 Acknowledgments .................................................................................................................... 4 Abstract ..................................................................................................................................... 5 Executive Summary .................................................................................................................. 7 Introduction ............................................................................................................................. 31 How this report was put together ........................................................................................... 36 Chapter Reports ...................................................................................................................... 41 Perdido Bay ........................................................................................................................... 41 Pensacola Bay .....................................................................................................................