Evolution, Ecology and Taxonomy of the Corydoradinae Revisited
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Cascadu, Flat-Head Or Chato
UWI The Online Guide to the Animals of Trinidad and Tobago Behaviour Callichthys callichthys (Flat-head Cascadu or Chato) Family: Callichthyidae (Plated Catfish) Order: Siluriformes (Catfish) Class: Actinopterygii (Ray-finned Fish) Fig. 1. Flat-head cascade, Callichthys callichthys. [“http://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=335”, Downloaded 10th October 2011] TRAITS. Was first described by Linnaeus in 1758 and was named Silurus callichthys. They generally reach a maximum length of 20 cm and can weigh up to 80g.(Froese and Pauly, 2001) The females are generally larger and more robust as compared to the males. The Callichthys callichthys is an elongated catfish with a straight or flattened belly profile. It also has a broad flattened head and a body which is almost uniform in breath with some posterior tapering which beings after dorsal fin. (Figure 2) Its body consists of 2 rows of overlapping plates or scutes. Approximately 26 – 29 scutes seen on the upper lateral series and 25 – 28 scutes seen on the lower lateral series. The fins are rounded and the fish also has a total of 6-8 soft dorsal rays. It also has 2 pairs of maxillary barbles near its mouth and small eyes. The fish has an inferior type mouth ( Berra, 2007). The Callichthys callichthys is dark olive green in colour to a grey brown as seen in Figure 1, with the males having a blue to violet sheen on its flanks. ECOLOGY. Callichthys callichthys is a freshwater organism which is primarily riverine in habitat (Arratia, 2003). Only 2 families of catfishes are found to colonise marine habitats UWI The Online Guide to the Animals of Trinidad and Tobago Behaviour (Arratia, 2003). -

Siluriformes: Callichthyidae) in the Subterranean Domain of Northern and Northeastern Brazil
13 4 297 Tencatt et al NOTES ON GEOGRAPHIC DISTRIBUTION Check List 13 (4): 297–303 https://doi.org/10.15560/13.4.297 First report of armored catfishes Callichthyinae Bonaparte, 1838 (Siluriformes: Callichthyidae) in the subterranean domain of northern and northeastern Brazil Luiz Fernando Caserta Tencatt,1 Bruno Ferreira dos Santos,2 Maria Elina Bichuette3 1 Universidade Estadual de Maringá, Coleção Ictiológica do Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura e Programa de Pós- Graduação em Ecologia de Ambientes Aquáticos Continentais, Av. Colombo, 5790, 87020-900 Maringá, Paraná, Brazil. 2 Universidade Federal de Mato Grosso do Sul, Programa de Pós-Graduação em Biologia Animal, Av. Costa e Silva, 79070-900 Campo Grande, Mato Grosso do Sul, Brazil. 3 Universidade Federal de São Carlos, Departamento de Ecologia e Biologia Evolutiva, Laboratório de Estudos Subterrâneos, Rodovia Washington Luis, km 235, 13565-905 São Carlos, São Paulo, Brazil. Corresponding author: Luiz Fernando Caserta Tencatt, [email protected] Abstract The first occurrence of the armored catfishes of the subfamily Callichthynae is reported in subterranean water bodies of northern and northeastern Brazil. The records include 3 species, each occurring in 1 of the 3 caves in the central and northeastern regions of Brazil: Callichthys callichthys from Casa do Caboclo cave, Sergipe state; Hoplosternum lit- torale from the Gruna da Lagoa do Meio, Bahia state; and Megalechis thoracata, from Casa de Pedra cave, Tocantins state. Keywords Camboatá, cave, hypogean habitat, karstic areas, Neotropical region. Academic editor: Bárbara Calegari | Received 2 March 2017 | Accepted 10 June 2017 | Published 14 August 2017 Citation: Tencatt LFC, Ferreira dos Santos B, Bichuette ME (2017) First report of armored catfishes Callichthyinae( Bonaparte, 1838) (Siluriformes: Callichthyidae) in the subterranean domain. -

Species Richness and Functional Structure of Fish Assemblages in Three Freshwater Habitats: Effects of Environmental Factors and Management
Received: 13 March 2019 Accepted: 26 July 2019 DOI: 10.1111/jfb.14109 REGULAR PAPER FISH Species richness and functional structure of fish assemblages in three freshwater habitats: effects of environmental factors and management Jamile Queiroz-Sousa1,2 | Sally A. Keith2,3 | Gianmarco S. David4 | Heleno Brandão5 | André B. Nobile1 | Jaciara V. K. Paes1 | Ana C. Souto1 | Felipe P. Lima1 | Reinaldo J. Silva1 | Raoul Henry1 | Katherine Richardson2 1Institute of Biosciences, São Paulo State University, Botucatu, Brazil Abstract 2Center for Macroecology, Evolution and In this study, the inverted trophic hypothesis was tested in the freshwater fish com- Climate, Natural History Museum of Denmark, munities of a reservoir. The distribution of fish species in three freshwater habitats in University of Copenhagen, Copenhagen, Denmark the Jurumirim Reservoir, Brazil, was examined using both species richness and the 3Lancaster Environment Centre, Lancaster relative proportions of different trophic groups. These groups were used as a proxy University, Lancaster, UK for functional structure in an attempt to test the ability of these measures to assess 4Agencia Paulista de Tecnologia do Agronegócio (APTA), Jaú, Brazil fish diversity. Assemblage structures were first described using non-metric multi- 5Paraná Federal Technology University, Santa dimensional scaling (NMDS). The influence of environmental conditions for multiple Helena, Brazil fish assemblage response variables (richness, total abundance and abundance per tro- Correspondence phic group) was tested using generalised linear mixed models (GLMM). The metric Jamile Queiroz-Sousa, Institute of Biosciences, typically employed to describe diversity; that is, species richness, was not related to São Paulo State University, Prof. Dr. Antônio Celso Wagner Zanin street, 250, 18618-689 - environmental conditions. -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Aspidoras Mephisto, New Species: the First Troglobitic Callichthyidae (Teleostei: Siluriformes) from South America
RESEARCH ARTICLE Aspidoras mephisto, new species: The first troglobitic Callichthyidae (Teleostei: Siluriformes) from South America Luiz Fernando Caserta Tencatt1*, Maria Elina Bichuette2 1 Departamento de Biologia, NuÂcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura, Programa de PoÂs-GraduacËão em Ecologia de Ambientes AquaÂticos Continentais, Universidade Estadual de MaringaÂ, MaringaÂ, ParanaÂ, Brazil, 2 Departamento de Ecologia e Biologia Evolutiva, LaboratoÂrio de Estudos SubterraÃneos, Universidade Federal de São Carlos, São Carlos, São Paulo, Brazil a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 a1111111111 Abstract Aspidoras mephisto n. sp. is described from the AneÂsio-Russão cave system, upper Tocan- tins River basin, GoiaÂs, Brazil. The species can be readily distinguished from its congeners OPEN ACCESS by troglomorphic features and also by presenting the following combination of features: Citation: Tencatt LFC, Bichuette ME (2017) infraorbital 1 generally with well-developed ventral laminar; or moderately developed; Aspidoras mephisto, new species: The first poorly-developed serrations on posterior margin of pectoral spine; nuchal plate not exter- troglobitic Callichthyidae (Teleostei: Siluriformes) nally visible; dorsal fin, even in conspicuously colored specimens, with only dark brown or from South America. PLoS ONE 12(3): e0171309. black chromatophores concentrated on rays, forming spots in some specimens; mem- doi:10.1371/journal.pone.0171309 branes hyaline; or sparse dark brown or black chromatophores on membranes, not forming Editor: Riccardo Castiglia, Universita degli Studi di any conspicuous pattern; and inner laminar expansion of infraorbital 1 moderately devel- Roma La Sapienza, ITALY oped. Information about its habitat, ecology, behaviour and conservation status are provided Received: October 11, 2016 and also a brief description of the juvenile stage. -

A New Aspidoras(Siluriformes: Callichthyidae) from Rio Paraguaçu
Neotropical Ichthyology, 3(4):473-479, 2005 Copyright © 2005 Sociedade Brasileira de Ictiologia A new Aspidoras (Siluriformes: Callichthyidae) from rio Paraguaçu basin, Chapada Diamantina, Bahia, Brazil Marcelo R. Britto*, Flávio C. T. Lima**, and Alexandre C. A. Santos*** During a recent ichthyological survey in Chapada Diamantina, Estado da Bahia, Brazil, a new, very distinctive Aspidoras was discovered in tributaries of the upper rio Paraguaçu. The new taxon differs from its congeners mainly in having: a poorly- developed pigmentation pattern, restricted to minute scattered blotches on dorsal region of head and body, but grouped in small, irregular blotches along the lateral body plate junction; four or five caudal vertebra, anterior to compound caudal centrum, with neural and haemal spines placed posteriorly, close to post-zygapophyses; and post-zygapophyses of the precaudal vertebrae without dorsal expansions connected with their respective neural spines. The new species shares with Aspidoras velites dorsolateral body plates not touching their counterparts dorsally, and infraorbital bones with reduced flanges that are restricted to the latero-sensory canal. Both of these are considered reductive character states, probably indicating a paedomorphic condition to both species. The new species is also compared to Aspidoras maculosus, a congener which bears the most similar color pattern and is geographically closest to the new species. Durante um estudo recente sobre a ictiofauna da Chapada Diamantina, foi descoberta uma nova espécie -

Redalyc.Dieta Do Cascudo Aspidoras Fuscoguttatus (Ostariophysi
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Casatti, Lilian; Veronezi Júnior, José Luis; de Paula Ferreira, Cristiane Dieta do cascudo Aspidoras fuscoguttatus (Ostariophysi, Callichthyidae) em riachos com diferentes características limnológicas e estruturais Biota Neotropica, vol. 9, núm. 1, enero-marzo, 2009, pp. 113-121 Instituto Virtual da Biodiversidade Campinas, Brasil Disponível em: http://www.redalyc.org/articulo.oa?id=199115787019 Como citar este artigo Número completo Sistema de Informação Científica Mais artigos Rede de Revistas Científicas da América Latina, Caribe , Espanha e Portugal Home da revista no Redalyc Projeto acadêmico sem fins lucrativos desenvolvido no âmbito da iniciativa Acesso Aberto Biota Neotrop., vol. 9, no. 1, Jan./Mar. 2009 Dieta do cascudo Aspidoras fuscoguttatus (Ostariophysi, Callichthyidae) em riachos com diferentes características limnológicas e estruturais Lilian Casatti1,2, José Luis Veronezi Júnior1 & Cristiane de Paula Ferreira1 1Laboratório de Ictiologia, Departamento de Zoologia e Botânica, IBILCE, Universidade Estadual Paulista – UNESP, Rua Cristóvão Colombo, 2265, Jardim Nazareth, CEP 15054-000, São José do Rio Preto, SP, Brasil, e-mail: [email protected], [email protected] 2Autor para correspondência: Lilian Casatti, e-mail: [email protected] CASATTI, L., VERONEZI JÚNIOR, J.L. & FERREIRA, C.P. Diet of the armored catfish Aspidoras fuscoguttatus (Ostariophysi, Callichthyidae) in streams with different limnological and structural features. Biota Neotrop. 9(1): http://www.biotaneotropica.org.br/v9n1/en/abstract?article+bn02109012009. Abstract: In the present study Aspidoras fuscoguttatus Nijssen & Isbrücker, 1976 diet was investigated based on specimens from 18 streams of São Paulo State northwestern region, upper Rio Paraná system. Stomach contents of 246 specimens were analyzed, with the registration of 26 types of feeding items showing high predominance of the autochthonous ones. -

ERSS-Corydoras Carlae
Corydoras carlae Ecological Risk Screening Summary U.S. Fish & Wildlife Service, August 2017 Revised, September 2017 Web Version, 11/30/2017 Photo: G. Ramsay. Licensed under Creative Commons, BY-NC. Available: http://www.fishbase.se/photos/UploadedBy.php?autoctr=12484&win=uploaded. (September 2017). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2017): “South America: Lower Iguazu River basin.” 1 Status in the United States This species has not been reported as introduced or established in the United States. Means of Introductions in the United States This species has not been reported as introduced or established in the United States. 2 Biology and Ecology Taxonomic Hierarchy and Taxonomic Standing From ITIS (2017): “Kingdom Animalia Subkingdom Bilateria Infrakingdom Deuterostomia Phylum Chordata Subphylum Vertebrata Infraphylum Gnathostomata Superclass Actinopterygii Class Teleostei Superorder Ostariophysi Order Siluriformes Family Callichthyidae Subfamily Corydoradinae Genus Corydoras Species Corydoras carlae Nijssen and Isbrücker, 1983” “Current Standing: valid” Size, Weight, and Age Range From Froese and Pauly (2017): “Max length : 5.4 cm SL male/unsexed; [Tencatt et al. 2014]” Environment From Froese and Pauly (2017): “Freshwater; demersal; pH range: 6.0 - 8.0; dH range: 2 - 25.” From Seriously Fish (2017): “Such habitats in Argentina [as where C. carlae has been found] are typically subject to significant seasonal variations in water volume, flow, turbidity, chemistry and temperature.” 2 Climate/Range From Froese and Pauly (2017): “Subtropical; 22°C - 26°C [Riehl and Baensch 1996], preferred ?” Distribution Outside the United States Native From Froese and Pauly (2017): “South America: Lower Iguazu River basin.” Introduced No introductions of this species have been reported. -
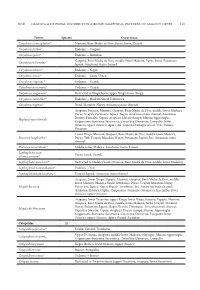
DAGOSTA & DE PINNA: DISTRIBUTION & BIOGEOGRAPHICAL PATTERNS of AMAZON FISHES Taxon Species Occurrence Corydoras Stenocep
2019 DAGOSTA & DE PINNA: DISTRIBUTION & BIOGEOGRAPHICAL PATTERNS OF AMAZON FISHES 113 Taxon Species Occurrence Corydoras stenocephalus* Mamoré, Beni-Madre de Dios, Purus, Juruá, Ucayali Corydoras sterbai* Endemic – Guaporé Corydoras sychri* Endemic – Marañon Guaporé, Beni-Madre de Dios, middle-lower Madeira, Purus, Juruá, Putumayo, Corydoras trilineatus* Japurá, Amazonas main channel Corydoras tukano* Endemic – Negro Corydoras urucu* Endemic – Coari-Urucu Corydoras virginiae* Endemic – Ucayali Corydoras weitzmani* Endemic – Ucayali Corydoras xinguensis* Restricted to Xingu basin (upper Xingu, lower Xingu) Corydoras zawadzkii* Endemic – Madeira Shield Tributaries Corydoras zygatus* Juruá, Marañon-Nanay, Amazonas main channel Araguaia, Juruena, Mamoré, Guaporé, Beni-Madre de Dios, middle-lower Madeira, Purus, Ucayali, Putumayo, Japurá, Negro, Amazonas main channel, Amazonas Estuary, Parnaíba, Capim, Araguari-Macari-Amapá, Maroni-Approuague, Hoplosternum littorale Coppename-Suriname-Saramacca, Corentyne-Demerara, Essequibo, lower Orinoco, upper Orinoco, Apure, Atl. Coastal Drainages of Col. Ven., Paraná- Paraguay Lower Xingu, Mamoré, Guaporé, Beni-Madre de Dios, middle-lower Madeira, Dianema longibarbis* Purus, Tefé, Ucayali, Marañon-Nanay, Putumayo, Japurá, Jari, Amazonas main channel Dianema urostriatum* Middle-lower Madeira, Amazonas main channel Lepthoplosternum Purus, Juruá, Ucayali altamazonicum* Lepthoplosternum beni* Restricted to Madeira basin (Mamoré, Beni-Madre de Dios, middle-lower Madeira) Lepthoplosternum stellatum* Endemic -

Redescription of Corydoras Guapore Knaack, 1961 (Siluriformes: Callichthyidae), a Midwater Corydoradinae Species from the Rio Guaporé Basin
Neotropical Ichthyology, 13(2): 287-296, 2015 Copyright © 2015 Sociedade Brasileira de Ictiologia DOI: 10.1590/1982-0224-20150018 Redescription of Corydoras guapore Knaack, 1961 (Siluriformes: Callichthyidae), a midwater Corydoradinae species from the rio Guaporé basin Luiz Fernando Caserta Tencatt1 and Carla Simone Pavanelli1,2 Corydoras guapore was described from the rio Guaporé, Rondônia State, Brazil, based only in three specimens, two of them merely examined alive in an aquarium and apparently not preserved posteriorly. The current location of these two paratypes is uncertain. In the original description, no standard diagnosis was presented and the descriptive information available is scarce and based only in external morphology. Thus, the aim of this study is to provide a redescription of C. guapore based in several topotypes. Corydoras guapore can be distinguished from its congeners by the presence of a short mesethmoid, with the anterior tip poorly developed; posterior margin of pectoral spine with conical serrations directed towards the origin of the spine; and by the lateral portion of caudal peduncle almost entirely blackened. Information about C. guapore ecology and conservation status are also provided. Corydoras guapore foi descrita do rio Guaporé, estado de Rondônia, Brasil, com base em somente três exemplares, sendo dois deles apenas examinados vivos em um aquário e aparentemente não preservados posteriormente. A atual localização desses dois parátipos é incerta. Na descrição original, uma diagnose padrão não foi apresentada e as informações descritivas disponíveis são escassas e baseadas apenas em morfologia externa. Dessa forma, o objetivo desse estudo é fornecer uma redescrição de C. guapore baseada em vários topótipos. -

PEIXES De RIACHOS Da Juréia-Itatins Cristina Gonçalves -- UNESP, São José Do Rio Preto, São Paulo Fotos Do Autor
Reserva Juréia-Itatins, Mata Atlântica, São Paulo, BRASIL 1 PEIXES de RIACHOS da Juréia-Itatins Cristina Gonçalves -- UNESP, São José do Rio Preto, São Paulo Fotos do autor. Um agradecimento especial a César Cestari e Ílson Prado pela assistência útil no campo. Produzido por: Tyana Wachter, R. Foster & Juliana Philipp. Com o apoio de Connie Keller e Andrew Mellon Foundation. © Cristina Gonçalves [[email protected]], UNESP, Instituto de Biociências, Letras e Ciências Exatas, Departamento de Zoologia e Botânica [fieldguides.fieldmuseum.org] [510] versão 2 05/2014 1 Astyanax ribeirae 2 Deuterodon iguape 3 Hollandichthys multifasciatus 4 Hyphessobrycon griemi lambari, piaba/characin lambari, piaba/characin lambari, piaba/characin lambarizinho/goldspotted tetra CHARACIFORMES CHARACIDAE CHARACIFORMES CHARACIDAE CHARACIFORMES CHARACIDAE CHARACIFORMES CHARACIDAE 5 Hyphessobrycon boulengeri 6 Mimagoniates microlepis 7 Oligosarcus hepsetus 8 Characidium lanei lambarizinho/characin lambari-azul, piaba-azul/blue tetra tajaba/characin mocinha, charutinho/south American darter CHARACIFORMES CHARACIDAE CHARACIFORMES CHARACIDAE CHARACIFORMES CHARACIDAE CHARACIFORMES CRENUCHIDAE 9 Characidium pterostictum 10 Characidium schubarti 11 Cyphocharax santacatarinae 12 Hoplias malabaricus mocinha, charutinho/south American darter mocinha, charutinho/south American darter saiguarú/toothless characin traíra/trahira CHARACIFORMES CRENUCHIDAE CHARACIFORMES CRENUCHIDAE CHARACIFORMES CURIMATIDAE CHARACIFORMES ERYTHRINIDAE 13 Phalloceros harpagos 14